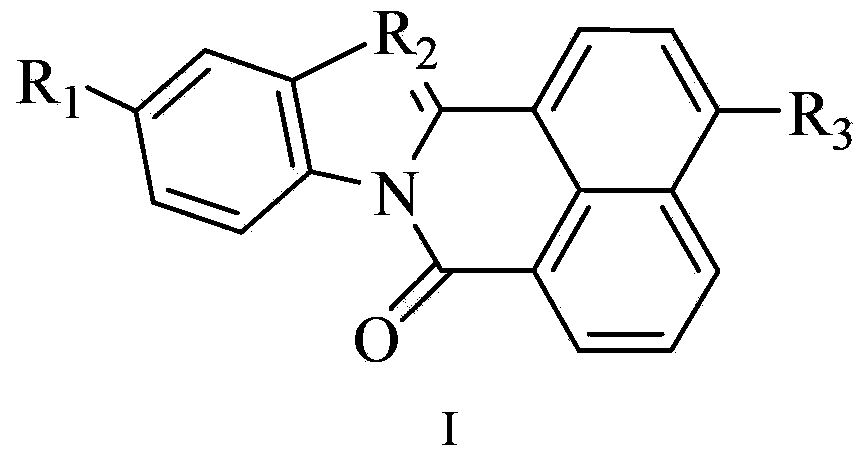
Two-photon fluorescent dye taking isoquinolinone as parent, preparation method thereof and application
A two-photon fluorescence, isoquinolinone technology, applied in luminescent materials, chemical instruments and methods, naphthalene dicarboxamide dyes/phthalimide dyes, etc., can solve the problem of reducing the application value of RNA dyes, poor photostability, and responsiveness Long time and other problems, to achieve the effect of high-efficiency two-photon absorption capacity, increase absorption cross-section, and increase π-electron conjugated system
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0042] 8. taking isoquinolinone as claimed in claim 1 is the preparation method of the two-photon fluorescent dye of parent, comprises the steps:
[0043] 1) 4-bromo-1,8-naphthalene anhydride reacts with the compound of formula i according to the molar ratio of 1:1-1:5 to prepare compound B:
[0044]
[0045] The reaction temperature is 70-150°C, the reaction time is 1-12 hours, and the reaction solvent is selected from dichloromethane, ethanol, ethyl acetate, acetic acid or a mixture thereof;
[0046] In a preferred embodiment, the reaction temperature is 80-140°C, the reaction time is 2-10 hours, the reaction solvent is selected from ethanol, ethyl acetate, acetic acid or a mixture thereof, 4-bromo-1,8-naphthalene anhydride and formula i Mole is 1:1-1:4;
[0047] In a further preferred embodiment, the reaction temperature is 90-120°C, the reaction time is 3-10 hours, the reaction solvent is selected from ethyl acetate, acetic acid or a mixture thereof, 4-bromo-1,8-naphth...
Embodiment 1
[0064] Embodiment 1. Preparation of dye compound A 1
[0065]
[0066] (1) Synthesis of Intermediate 1
[0067] Add 20mmol of 4-bromo-1,8-naphthalene anhydride and 35mmol of 4-nitro-o-phenylenediamine into a round bottom flask containing 20ml of acetic acid solution under nitrogen protection. The reaction was heated to reflux at 105°C for 3 hours and then stopped. The mixture was poured into ice water, precipitated out, and suction filtered to obtain the crude product of yellow solid powder, intermediate product 1, with a yield of 84%.
[0068] (2) Dye A 1 Synthesis
[0069] Add 20 mmol of crude product 1 and 25 mmol of ethanolamine from the previous step into a round-bottomed flask containing 20 ml of ethylene glycol monomethyl ether solution, under nitrogen protection. The reaction was heated to reflux at 125°C for 4 hours and then stopped. The mixture was poured into ice water, an orange-red precipitate was precipitated, and the crude product was obtained by suctio...
Embodiment 2
[0070] Example 2. Dye Compound A 1 Labeling assay on cellular RNA
[0071] Compound A synthesized using Example 1 1 , at a concentration of 5 μM A 1 - Add 4 μL of DMSO solution to the Hela cells at 37°C, 5% CO 2 dye A 1 Hela cells were incubated in culture medium for 30 minutes. Then, shake and rinse with PBS for 5min×3, then add cell culture medium, and conduct two-photon laser confocal imaging. Select a representative area, observe with an oil lens (60×), and repeat three times. Experimental results such as figure 2 As shown, the imaging shows that there is a strong fluorescent signal in the cellular RNA of Hela cells, while there is no fluorescent signal in other regions of the cell. figure 2 For adding dye A 1 Focused image of posterior HeLa cells. The excitation wavelength is 800nm, and the collection wavelength range is 520-570nm. The scale bar in the figure is 20 μm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com