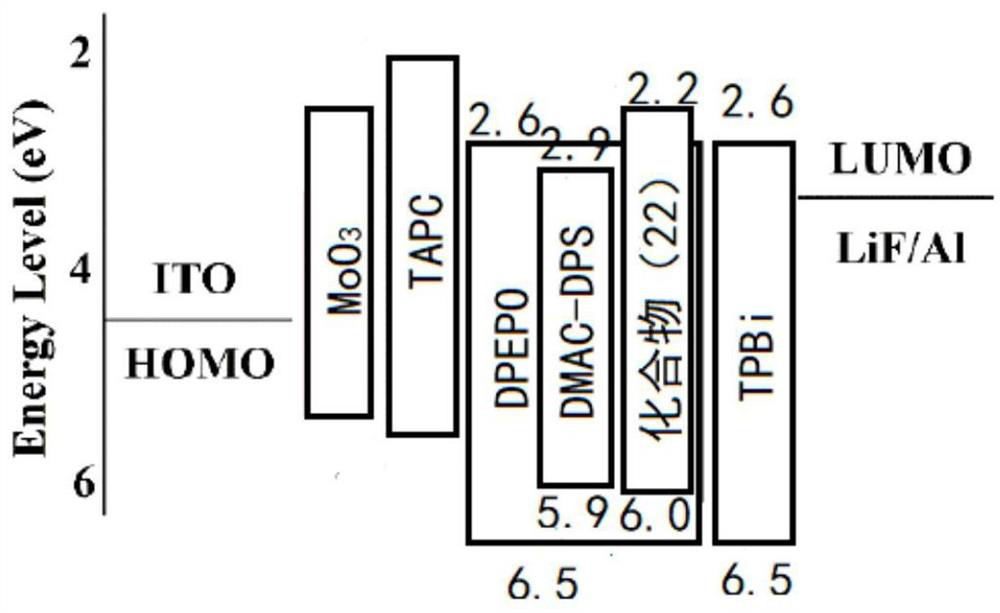
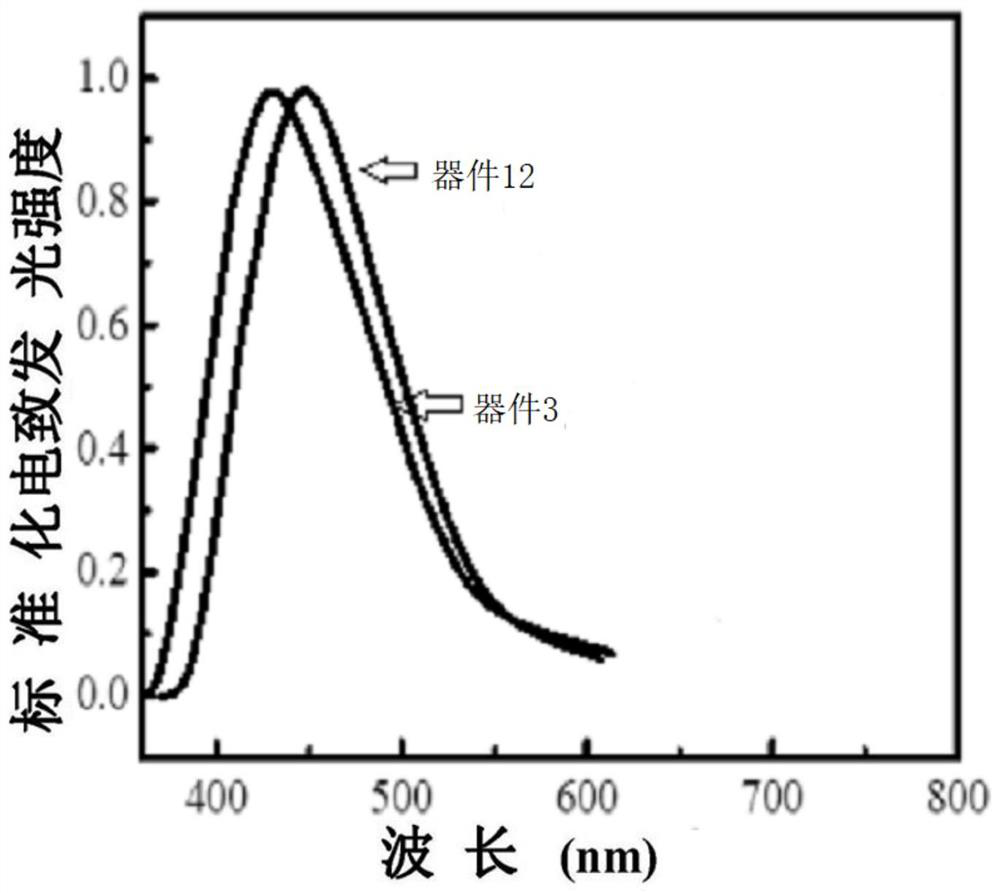
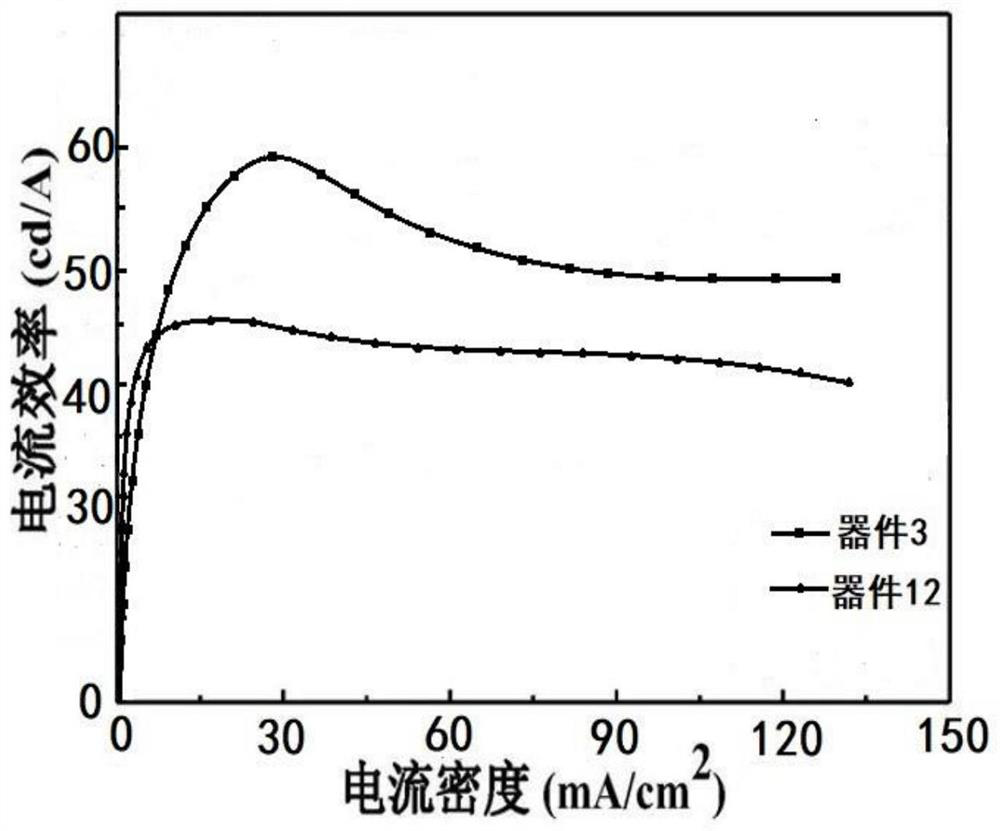
A thermally induced delayed fluorescent material and its preparation method and application
A technology of thermally induced delayed fluorescence and reactants, which is applied in the fields of luminescent materials, chemical instruments and methods, semiconductor/solid-state device manufacturing, etc., can solve the problems of poor performance and efficiency roll-off of TADF devices, so as to avoid non-radiative transitions and expand distance, improving the effect of device efficiency roll-off
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Embodiment 1: the present invention is that above-mentioned compound 1 can be synthesized by the following method:
[0061]
[0062] (1) In a 1L reaction flask, add 2-chloro-10H-phenothiazine (46.74g, 200mmol), bromobenzene (31.40g, 200mmol), 1,2-cyclohexanediamine (2.28g, 20mmol), Cuprous iodide (0.76g, 4mmol), sodium tert-butoxide (38.44g, 400mmol), 500g 1,4-dioxane, under the protection of nitrogen, heated up to 95°C, reacted for 10h, liquid phase monitoring completed the reaction, Cool down to stop the reaction. Filter the reaction solution with a silica gel funnel, wash the filtrate with water, separate layers, concentrate, and beat once with 1:10 petroleum ether and ethyl acetate to obtain 48.95 g of intermediate b with a yield of 79%;
[0063] (2) In a 1L reaction flask, add intermediate b (37.18g, 120mmol), dichloromethane (250mL), hydrogen peroxide (H 2 o 2 ) (25mL), acetic acid (AcOH) (125mL), the temperature was raised to 70°C, and the reaction was carr...
Embodiment 2
[0066] Embodiment 2: The present invention is that the above-mentioned compound 10 can be synthesized by the following method.
[0067]
[0068] (1) In a 1L reaction flask, add 2-chloro-10H-phenothiazine (46.74g, 200mmol), 1-bromodibenzofuran (49.42g, 200mmol), 1,2-cyclohexanediamine (2.28 g, 20mmol), cuprous iodide (0.76g, 4mmol), sodium tert-butoxide (38.44g, 400mmol), 500g 1,4-dioxane, under the protection of nitrogen, the temperature was raised to 95°C, and the reaction was carried out for 10h. The phase monitoring reaction is completed, and the temperature is lowered to stop the reaction. Filter the reaction solution with a silica gel funnel, wash the filtrate with water, separate layers, concentrate, and beat once with 1:10 petroleum ether and ethyl acetate to obtain 59.98 g of intermediate b with a yield of 75%;
[0069] (2) In a 1L reaction flask, add intermediate b (47.98g, 120mmol), dichloromethane (400mL), hydrogen peroxide (H 2 o 2 ) (40mL), acetic acid (AcOH...
Embodiment 3
[0072] Example 3: The present invention is that the above-mentioned compound 21 can be synthesized by the following method.
[0073]
[0074] (1) In a 1L reaction flask, add 2-chloro-10H-phenothiazine (46.74g, 200mmol), 9-(4-bromophenyl)-9H-carbazole (64.44g, 200mmol), 1,2 -cyclohexanediamine (2.28g, 20mmol), cuprous iodide (0.76g, 4mmol), sodium tert-butoxide (38.44g, 400mmol), 500g 1,4-dioxane, under nitrogen protection, the temperature was raised to 95°C, react for 10h, monitor the completion of the reaction by liquid phase monitoring, and stop the reaction by lowering the temperature. Filter the reaction solution with a silica gel funnel, wash the filtrate with water, separate layers, concentrate, and beat once with 1:10 petroleum ether and ethyl acetate to obtain 73.15 g of intermediate b with a yield of 77%;
[0075] (2) In a 1L reaction flask, add intermediate b (57.00g, 120mmol), dichloromethane (500mL), hydrogen peroxide (H 2 o 2 ) (50 mL), acetic acid (AcOH) (2...
PUM
| Property | Measurement | Unit |
|---|---|---|
| external quantum efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com