Tropinone derivative and its preparation method and application
A kind of technology of tropinone derivative, acetoxy tropane, applied in the field of preparation of new compound
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] The synthesis of embodiment 1 genipin derivative
[0036] 1.1 Instruments and reagents
[0037] Nuclear magnetic resonance spectrum adopts Brucker400MHz ADVANCE DMX500 type nuclear magnetic resonance instrument to measure (TMS is internal standard, CDC1 3 is the solvent); ESI mass spectrum was determined by Esquire600 (Brucker, Germany) mass spectrometer.
[0038] The column chromatography silica gel is 200-300 mesh (Qingdao Ocean Chemical Factory), and the chemical reagents are domestic analytical reagents without further purification before use.
[0039] 1.2 Preparation of tropin ketone
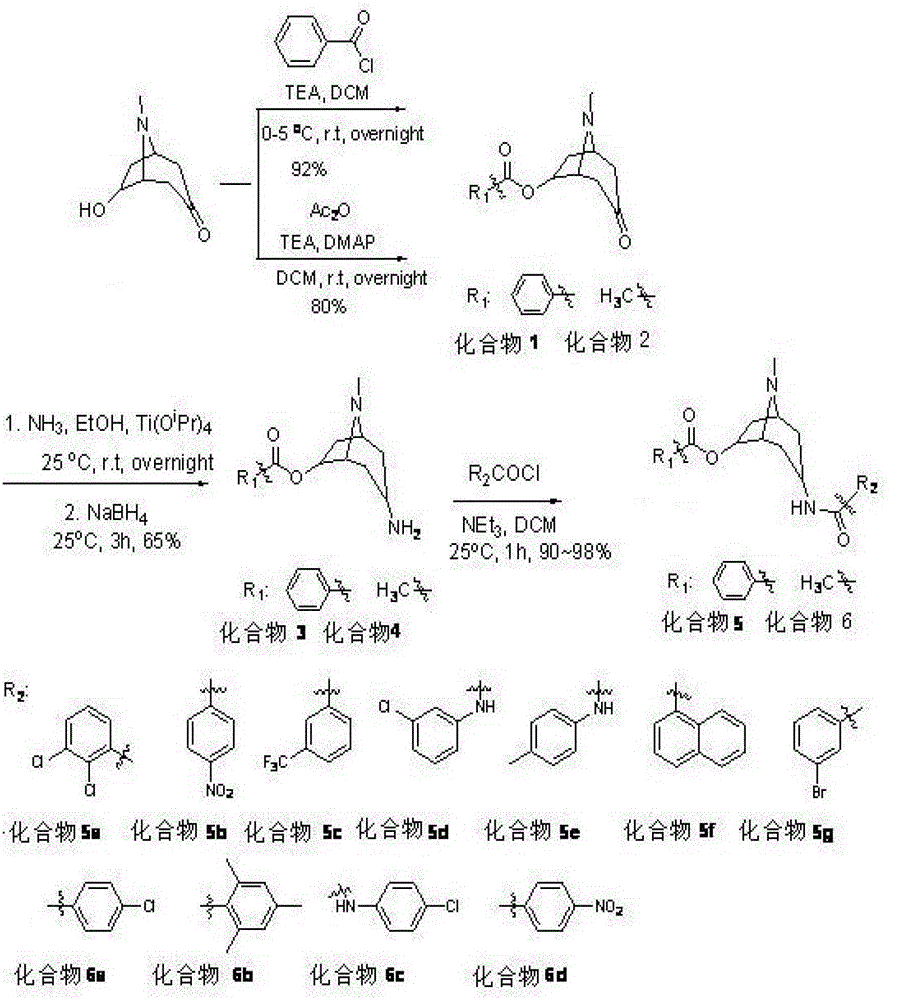
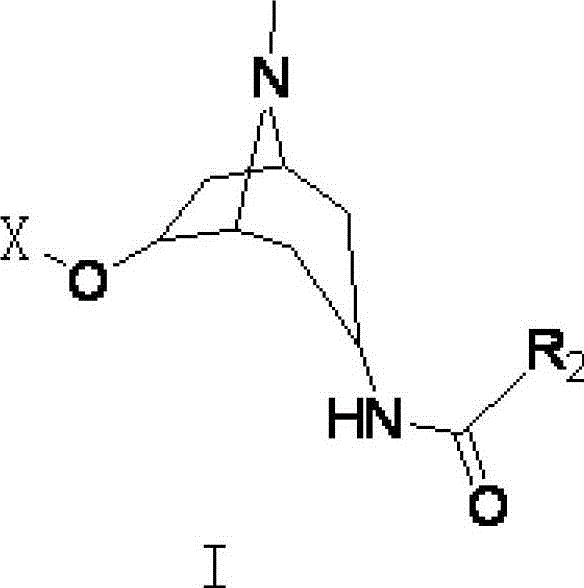
[0040] Compound 1 (see attached figure 1 ) preparation: Add 5.00g of 6-hydroxytropinone, 50mL of dichloromethane, and 3mL of triethylamine in a 250mL three-neck flask, and add 5mL of benzoyl chloride dropwise under nitrogen protection in an ice bath, and stir overnight at room temperature. The solvent was removed by rotary evaporation under reduced pressure, and the residue was ...
Embodiment 2
[0058] Example 2 Anti-diabetic bioactivity detection of tropinone derivatives
[0059] Dipeptidyl peptidase IV inhibitors are new hypoglycemic drugs that have attracted much attention at present. Studies have shown that dipeptidyl peptidase IV inhibitors can promote the release of insulin from β cells, increase the synthesis of insulin in β cells, and inhibit the secretion of glucagon at the same time, without hypoglycemia events and fewer side effects. Type 2 diabetes has very good prospects.
[0060] 2.1 Experimental reagents
[0061] Rosiglitazone (Sigma, R2408)
[0062] TransIT-293 Transfection Reagent (Mirus, MIR2706)
[0063] Dimethylsulfoxide (Sigma, Cat.No.154938-1L, Lot.No.52396AK)
[0064] 384-well plate (384-magnetic separator) (Greiner, 781080)
[0065] Dipeptidyl peptidase IV (Sigma, Cat#D4943, Lot#080M1723)
[0066] Dipeptidyl peptidase IV – globulin TM Protease (Promega, Cat#G8351, Lot#320119)
[0067] 384-well plate (PerkinElmer, OptiPlate-384, White Op...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com