Synthesis method of tropinone
A synthesis method and tropinone technology are applied in the fields of synthetic biology and enzyme catalysis, which can solve the problems of insufficient stability and low expression, and achieve the effects of increasing yield and high application value.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
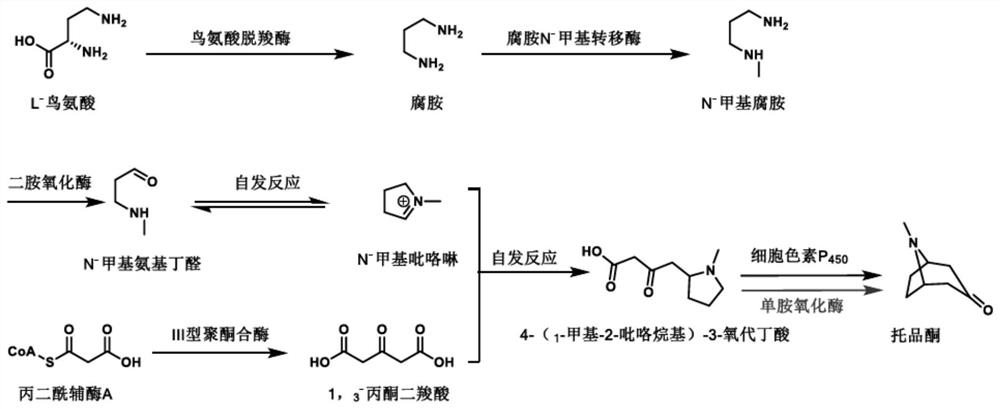
[0029] Example 1: Fermentative accumulation of engineering strain PL1-AaPKS 4-(1-methyl-2-pyrrolidinyl)-3-oxobutanoic acid
[0030] Take out the engineering strain PL1-AaPKS from the -80°C refrigerator, inoculate it into 5mL YPD medium, culture it in a shaker at 30°C (rotating speed 200rpm) for 2-3 days, and inoculate it to 100mL YPD according to the inoculation amount of 10%-15%. The culture medium was cultured at 30°C for 5 days, and the fermentation supernatant was collected by centrifugation, freeze-dried and concentrated to the original volume of 5-10 mL.
Embodiment 2
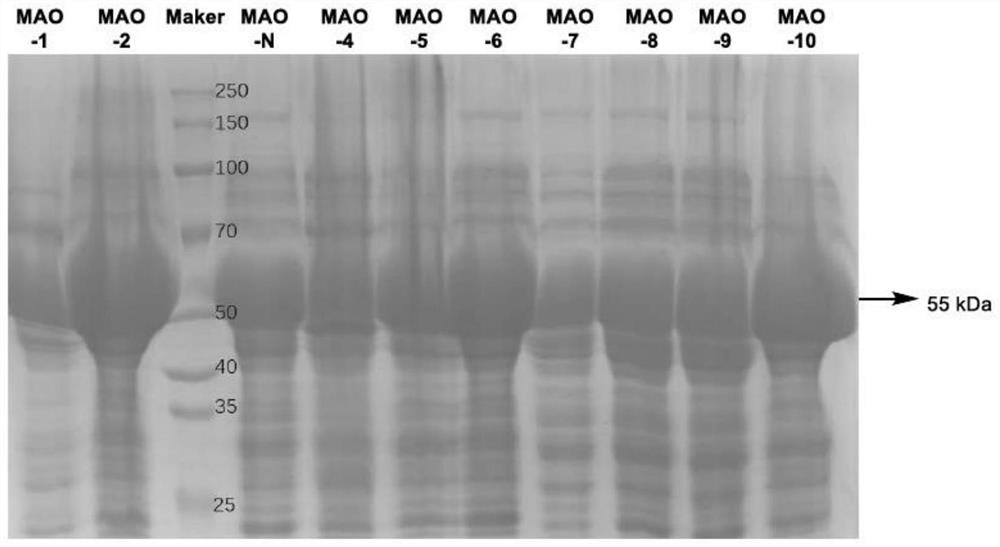
[0031] Embodiment 2: the crude enzyme liquid freeze-dried powder preparation of monoamine oxidase
[0032] The synthesis of the monoamine oxidase gene sequence (shown in SEQ ID NO.2) and the construction of the monoamine oxidase expression plasmid pET28a-MAO-N were completed by Jinweizhi Biotechnology Co., Ltd. Take Escherichia coli competent cells E.coli BL21 out of the -80°C refrigerator and place them on ice, pipette 1 microliter of the constructed monoamine oxidase expression plasmid pET28a-MAO into the competent cells, mix gently, Ice-bathed for 30 minutes, then placed in a 42°C water bath for heat shock for 90 seconds, immediately placed on ice for 2 minutes, and added 1 mL of LB to the transformation system. After cultivating on a shaker at 37°C for 40-60 minutes, take out the low-speed centrifugation for 3 minutes, discard most of the supernatant, gently blow the resuspended bacteria with a pipette gun, and spread it on the LA plate containing 50 μg / mL kanamycin antibi...
Embodiment 3
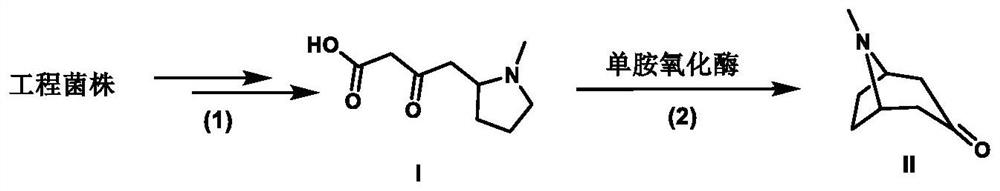
[0035] Example 3: Biotransformation of 4-(1-methyl-2-pyrrolidinyl)-3-oxobutanoic acid to produce tropinone
[0036] Add monoamine oxidase crude enzyme lyophilized powder to 200 μL of the fermented broth concentrated in Example 1 to a final concentration of 50 μg / mL, and at the same time add FAD to a final concentration of 1 mM, and peroxidase to a final concentration of 25 μg / mL; ~7 days, lyophilize the reaction solution, resuspend with 200 μL acetonitrile, centrifuge to get the supernatant and detect the formation of tropinone by LC-MS (MS detection results are as follows: Figure 4 ), the results showed that the conversion rate of this reaction was as high as 90%.
[0037] LC-MS detection conditions:
[0038] Instrument: SHIMADZU LCMS-2020UPLC-MS;
[0039] Analytical column: R227-32015-03Shim-pack Velox Biphenyl (2.7μm, 2.1x100 mm, Shimadzu Corporation), the flow rate is 0.4mL min -1 , the detected mobile phase is (A = deionized water, B = CH containing 0.1% formic acid ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com