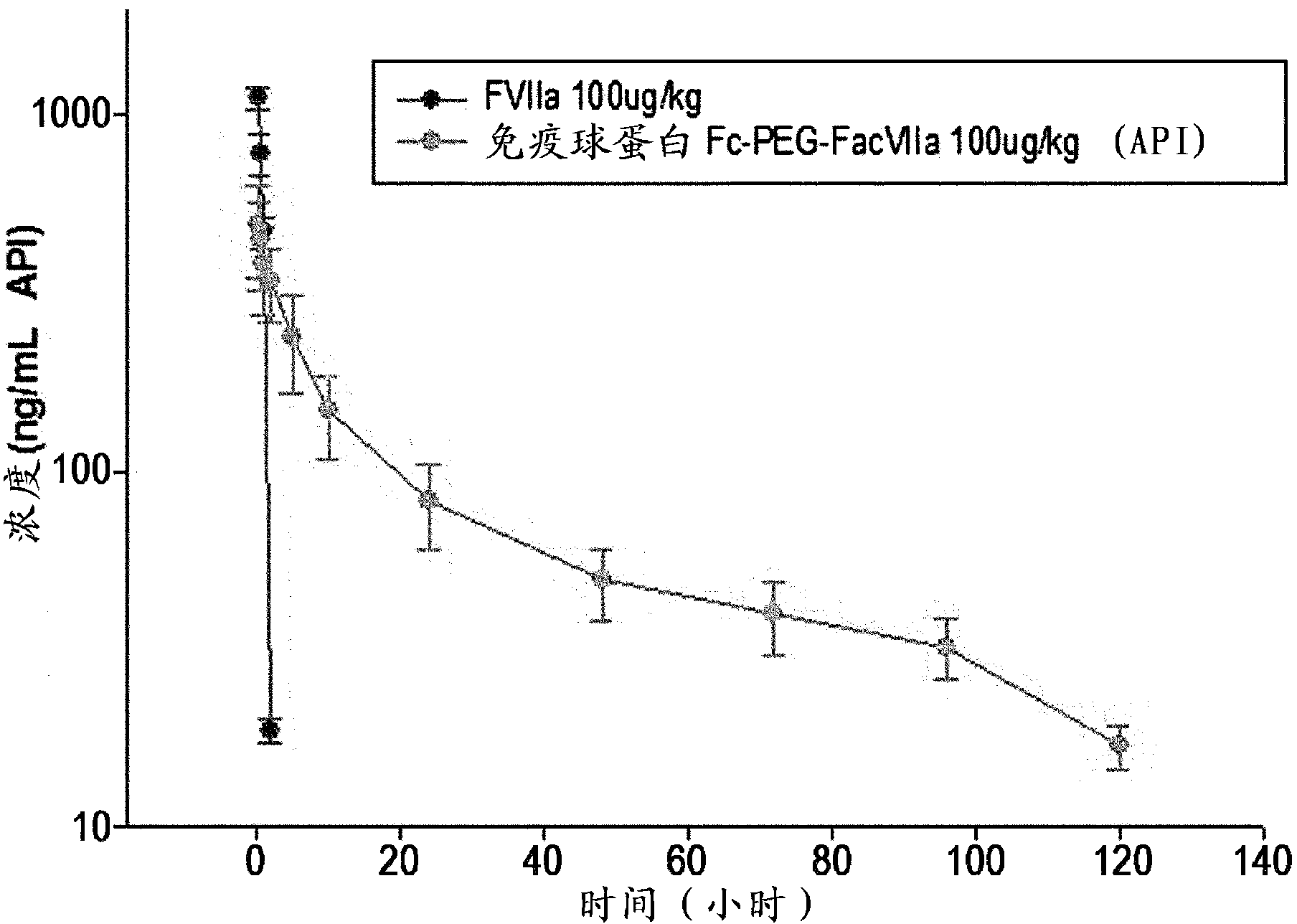
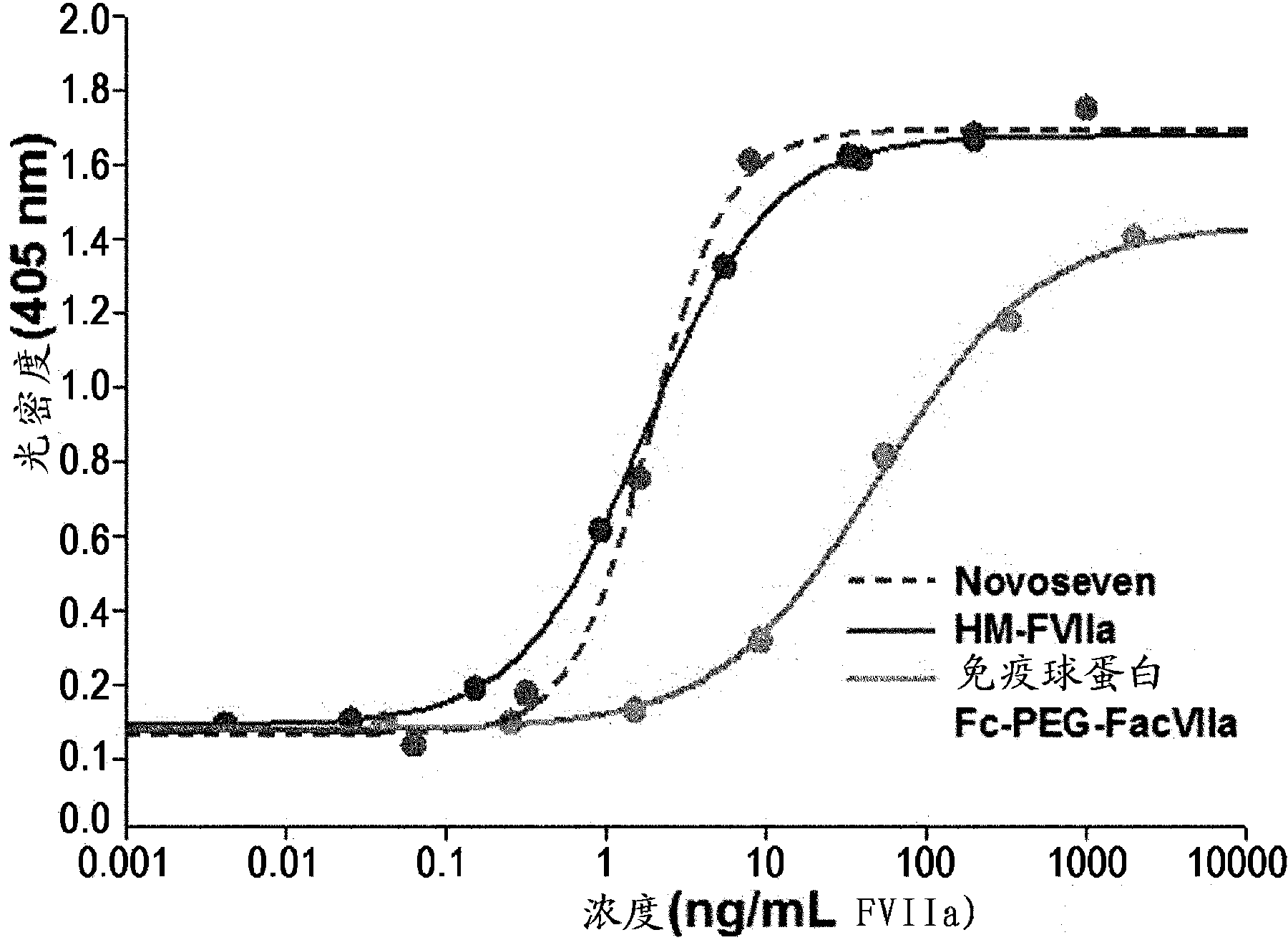
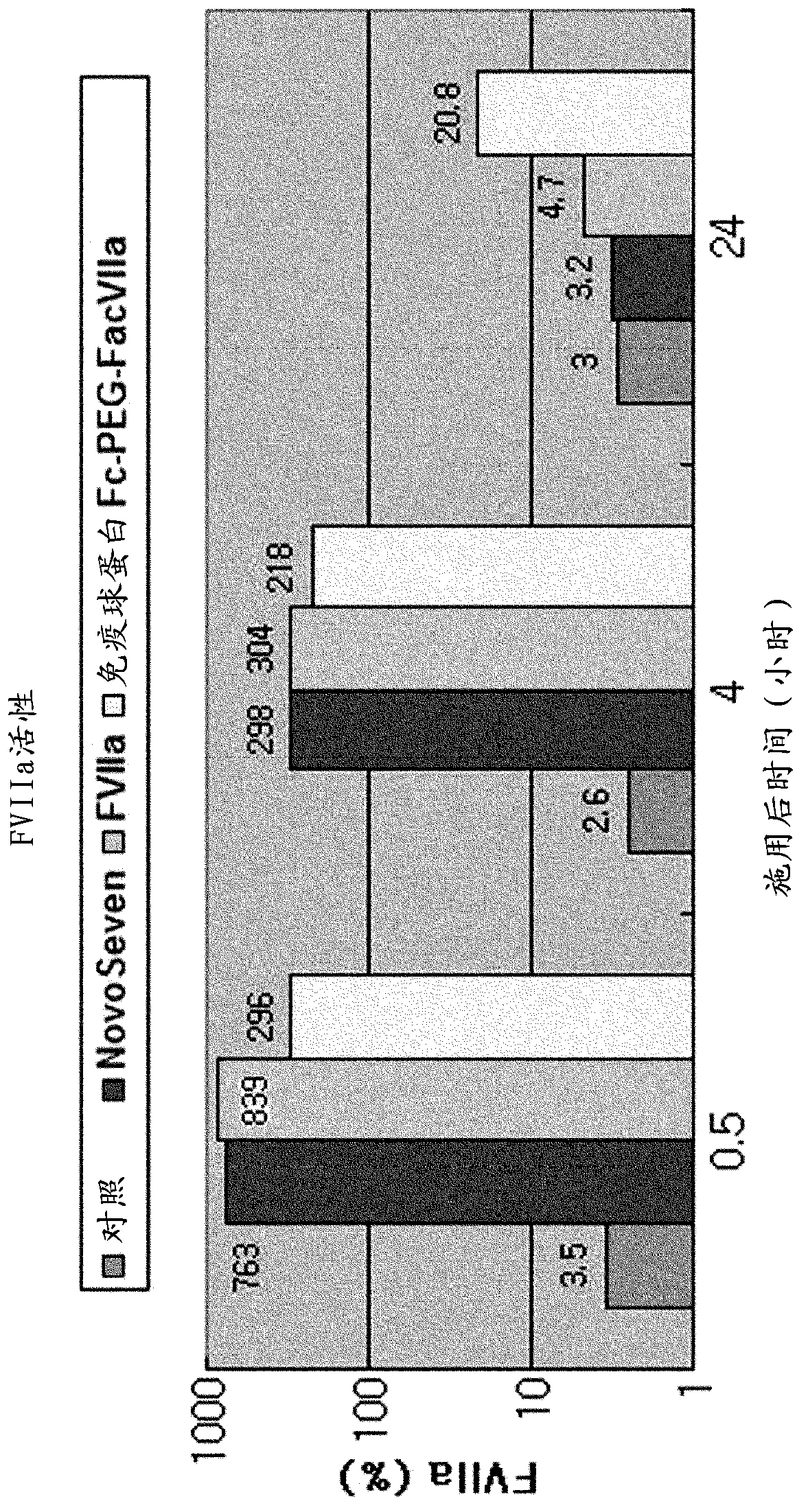
Factor viia complex using an immunoglobulin fragment
A technology of immunoglobulin and compound, which is applied in anti-animal/human immunoglobulin, coagulation/fibrinolytic factors, animal/human protein, etc., can solve the problems of insufficient prevention and treatment of hemophilia, and achieve prolonged serum Half-life, compliance-enhancing effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0090] Example 1: Preparation of Immunoglobulin Fc-PEG-FacVIIa Complex
[0091] Immunoglobulin Fc was pegylated at the N-terminus with 5K propionaldehyde (3) PEG (PEG with three terminal propionaldehyde groups, NOF, Japan). In this regard, 6 mg / mL of immunoglobulin Fc was reacted with PEG at 4°C for 4.5 hours, and the molar ratio of immunoglobulin Fc to PEG was set at 1:2. The reaction was performed in 100 mM potassium phosphate buffer at pH 6.0 in 20 mM SCB (NaCNBH 3 ) in the presence of a reducing agent. The reaction mixture was loaded onto SOURCEQ (LRC 2585ml, Pall Corporation) to purify mono-PEGylated immunoglobulin Fc. Then, FVII was coupled to immunoglobulin Fc-5K PEG at a molar ratio of 1:10 (FVII:immunoglobulin Fc-5K PEG) at 4° C. for 18 hours, and the total protein concentration was set at 20 mg / mL. Coupling reactions were performed in 100 mM potassium phosphate, pH 6.0, in the presence of 20 mM SCB as reducing agent. The coupling reaction mixture was purified by ...
Embodiment 2
[0102] Example 2: Preparation of 20k PEG-FacVIIa(N) Conjugate
[0103]FVII (FacVII) was pegylated at the N-terminus with 20K mPEG butyraldehyde (Nektar, USA). In this regard, 5 mg / mL of FVII was reacted with PEG at 4° C. for 10 hours, and the molar ratio of FVII to 20K PEG was set at 1:5. The reaction was performed in 100 mM sodium acetate buffer at pH 5.0 in 20 mM SCB (NaCNBH 3 ) in the presence of a reducing agent. Mono-PEGylated FVII was purified by RESOURCE Q (1 ml, prepackaged, GE Healthcare). Given a salt gradient of 1 M NaCl in 20 mM Tris (pH 7.5), the column eluted polyPEGylated FVII, monoPEGylated FVII and FVII sequentially. Then, a second purification was performed using a Superdex_200 (Hiroad 16 / 60, GE Healthcare) column to separate mono-PEGylated FVII from FVII and FVII multimer impurities. For activation, mono-PEGylated FVII was reloaded onto SOURCE Q, followed by pouring into a mobile phase containing 1.75 mM calcium ions for 1 hour. Elution was performed wi...
Embodiment 3
[0113] Example 3: Preparation of 20K PEG-FacVIIa(Lys) Conjugate
[0114] FVII was pegylated at lysine residues with 20k mPEG SPA (Nektar, USA). In this regard, 3 mg / mL of FVII was reacted with 20k PEG for 3 hours at room temperature, and the molar ratio of FVII to 20k PEG was set at 1:5. The reaction was performed in 100 mM sodium phosphate buffer, pH 8.0. Mono-PEGylated FVII was purified by RESOURCE Q (1 ml, prepackaged, GE Healthcare). Given a salt gradient of 1 M NaCl in 20 mM Tris (pH 7.5), the column eluted polyPEGylated FVII, monoPEGylated FVII and FVII sequentially. Then, a second purification was performed with a Superdex_200 (Hiroad 16 / 60, GE Healthcare) column to separate monoPEGylated FVII from FVII and FVII multimer impurities. For activation, purified mono-PEGylated FVII was reloaded onto SOURCE Q, followed by pouring on the column with a mobile phase containing 1.75 mM calcium ions for 1 hour. Elution was performed with 35 mM calcium ions to obtain monoPEGyla...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap