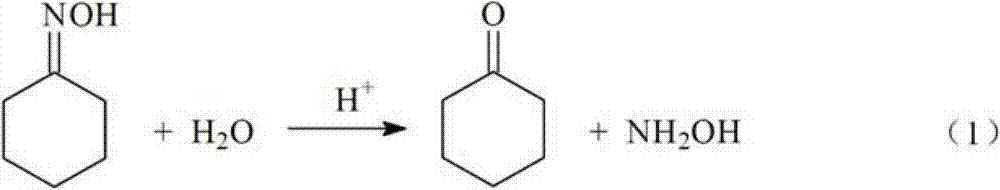
A kind of method that catalyzes cyclohexanone oxime hydrolysis reaction in acidic ionic liquid
An acidic ionic liquid, cyclohexanone oxime technology, applied in chemical instruments and methods, preparation of organic compounds, catalysts for physical/chemical processes, etc. , Overcome the effect of corrosion equipment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
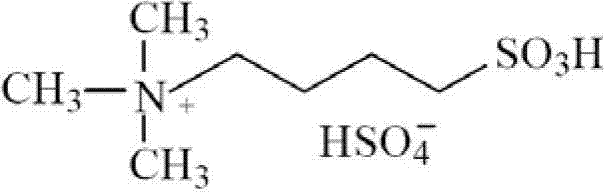
[0023] The molecular structure of the N,N,N-trimethyl-N-sulfobutyl ammonium bisulfate ionic liquid described in this embodiment is as follows:
[0024]
[0025] Referring to the synthesis method reported in the literature (Acta Chemical Society, 2009, 60(2): 345-350), equimolar trimethylamine aqueous solution (≥33%) and 1,4-butane sultone were added to the reaction flask, and React in a water bath at 50°C for 12 hours to obtain a white solid; add equimolar concentrated sulfuric acid (≥98.0%) after purification and drying, react in a water bath at 80°C for 4 hours to obtain a colorless or light yellow transparent liquid, wash with ether, and dry in vacuo Then the target ionic liquid is obtained.
Embodiment 2
[0027] The molecular structure of the N,N,N-trimethyl-N-sulfobutyl trifluoromethanesulfonate ionic liquid described in this example is as follows:
[0028]
[0029] Same as the operating steps and reaction conditions of Example 1, just after the purification and drying steps, the original step of "adding equimolar concentrated sulfuric acid" is replaced by "adding equimolar trifluoromethanesulfonic acid", after the reaction is completed, The target ionic liquid was obtained by washing with ether and drying in vacuum.
Embodiment 3
[0031] The molecular structure of the 1-sulfobutyl-3-methylimidazolium trifluoromethanesulfonate ionic liquid described in this example is as follows:
[0032]
[0033] Referring to the synthesis method reported in the literature (Chinese Journal of Chemical Engineering, 2009, 17(5):756-760), equimolar 1-methylimidazole and 1,4-butane sultone were added to the reaction flask, and React in a water bath at 40°C for 12 hours to obtain a white solid; add equimolar trifluoromethanesulfonic acid after purification and drying, and react in a water bath at 80°C for 6 hours to obtain a colorless or light yellow transparent liquid, which is washed with ether and dried in vacuum. to obtain the target ionic liquid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com