Assembly to facilitate user reconstitution
A technology of components and kits, applied in special packaging objects, packaged food, packaging, etc., can solve problems such as difficulties for patients or medical staff during the reorganization process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0034] The present disclosure provides recombination components particularly useful for reconstituting lyophilized pharmaceuticals. While the assembly is primarily described herein with reference to the reconstitution of lyophilized pharmaceuticals, it will be apparent that the assembly can be used to reconstitute other materials.
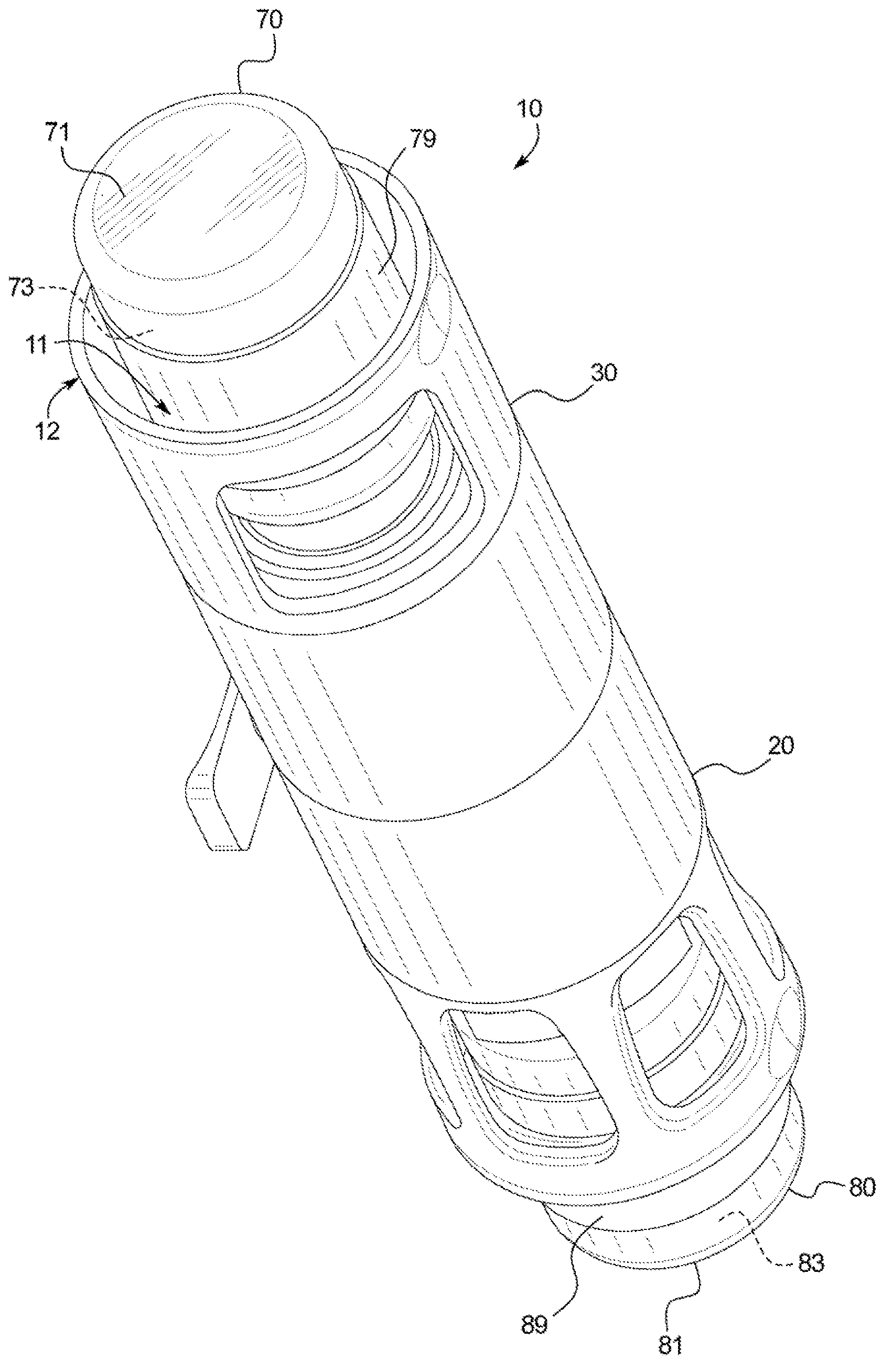
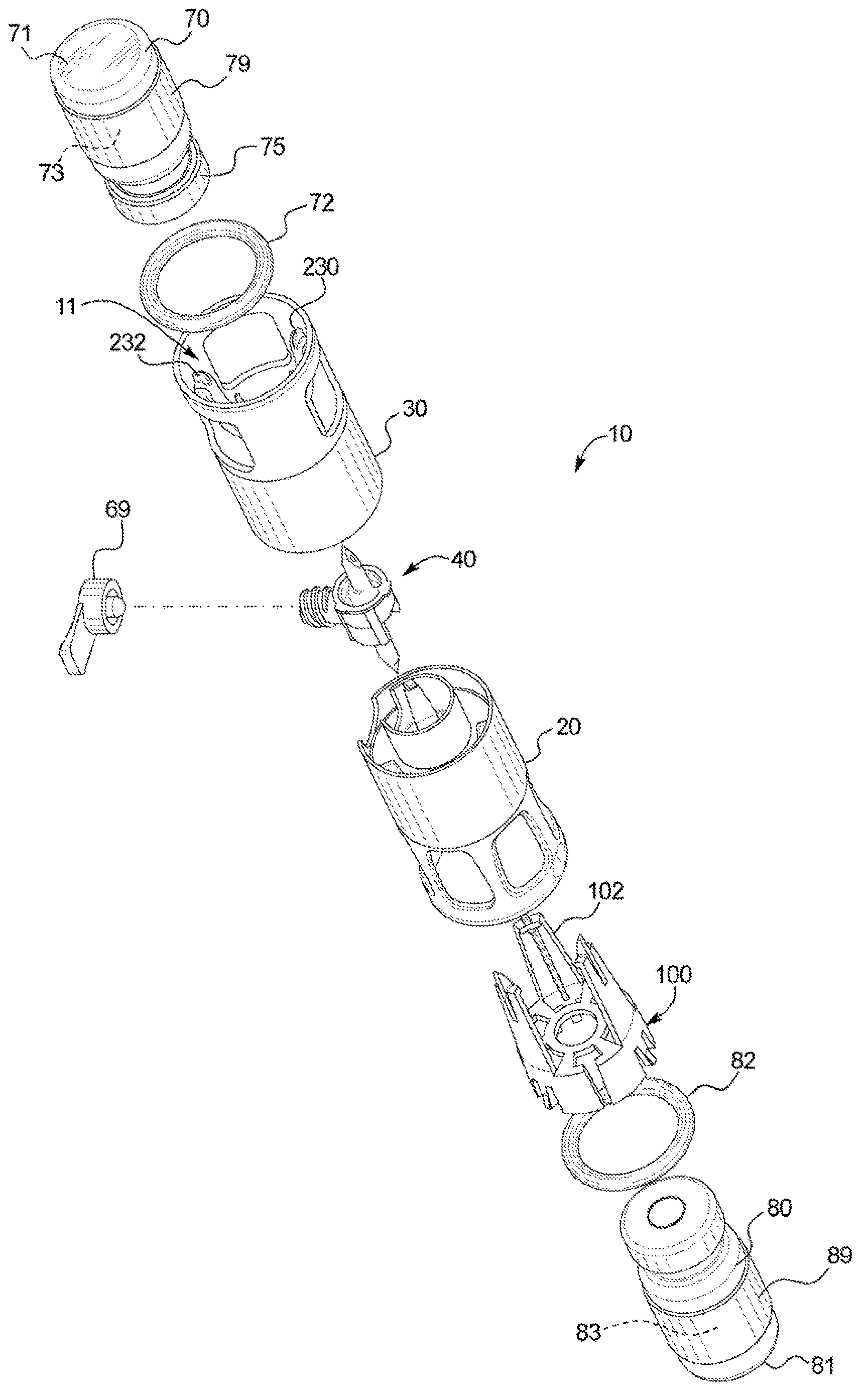
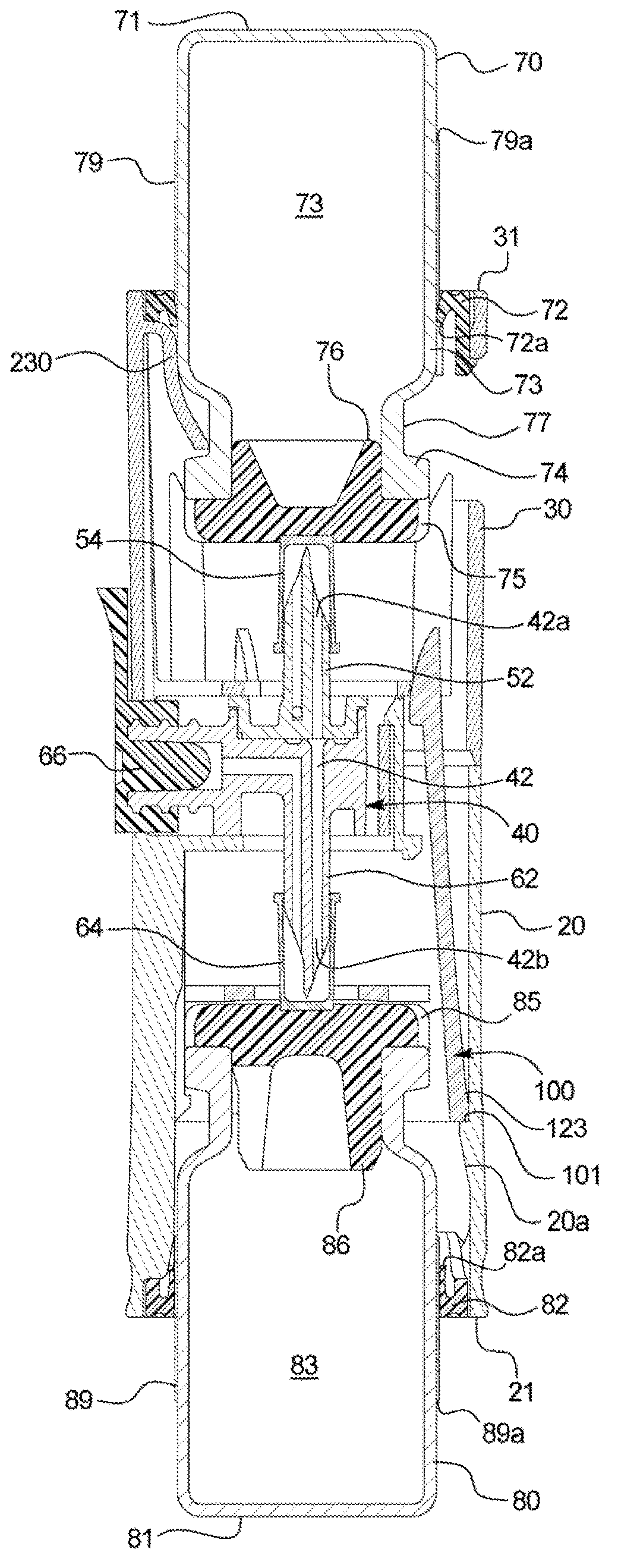
[0035] Referring now to the attached drawings and specifically to figure 1 and 2 , a recombination assembly 10 is shown. Assembly 10 includes a housing 12 . Housing 12 maintains alignment and restricts movement of internal components. Housing 12 includes a first or lower sleeve 20 and a second or upper sleeve 30 and defines a generally cylindrical interior passage 11, and at least a portion of a second container 80 is disposed between first or lower sleeve 20 and Channel 11. Housing 12 may be surrounded by packaging during storage and transport.
[0036] Transfer Kit 40 ( figure 2 ) is disposed within the housing 12 and fixed between the co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com