Recombinant expression of proteins in a disulfide-bridged, two-chain form
A disulfide bridge, protein technology, applied in the direction of peptide/protein composition, recombinant DNA technology, fusion with protease sites, etc., can solve the problems of difficult separation, can not be excluded under any circumstances, cost and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
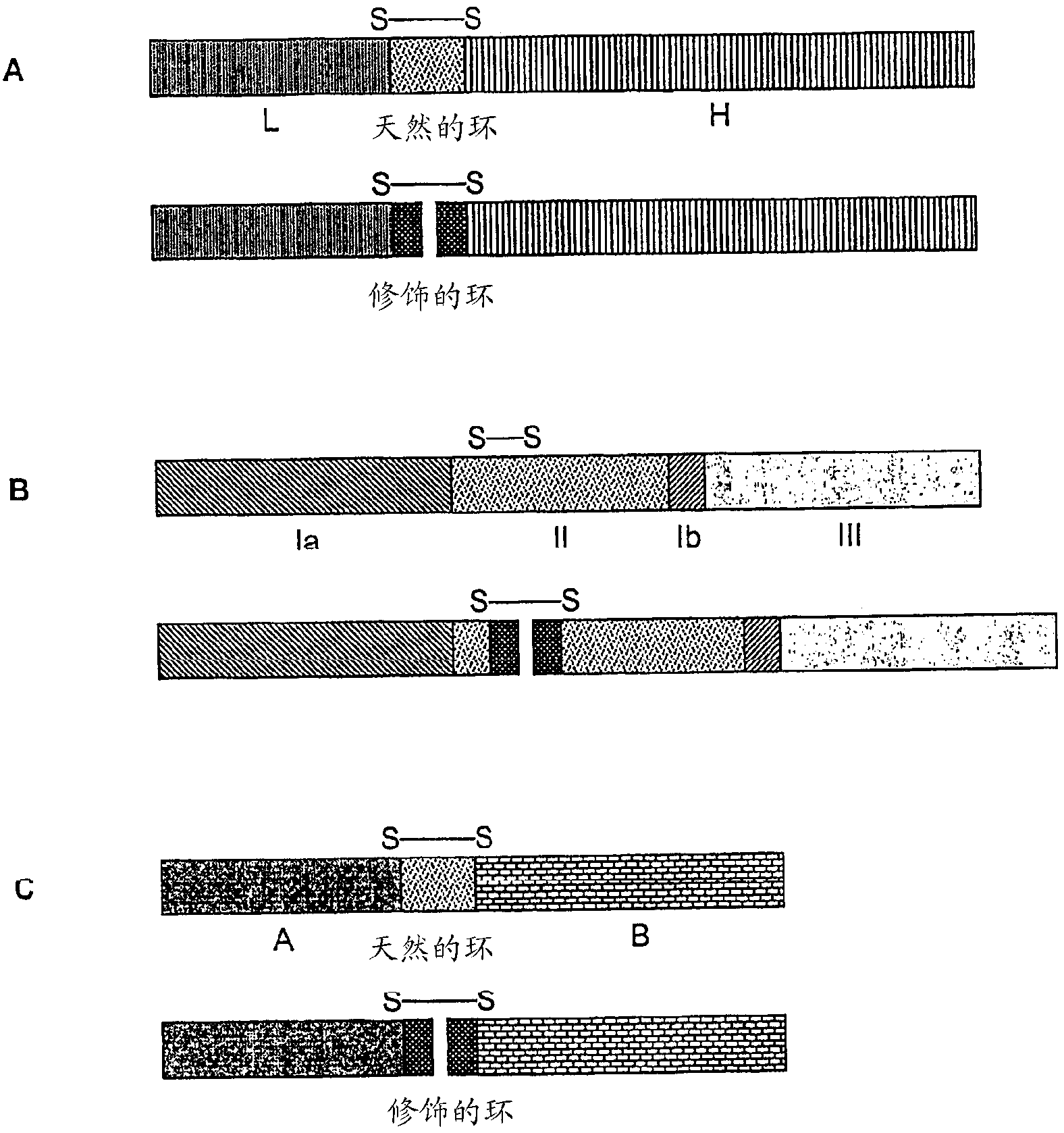
[0084] According to the invention, the first chain of the protein / polypeptide is preferably the chain encoded by the N-terminus of the corresponding DNA, whereas the second chain of the protein / polypeptide is thus the chain encoded by the C-terminus of the corresponding DNA. Since the expression of the 5'-DNA-3' results in N-polypeptide-C, in the aforementioned preferred case of the invention, this means that said expression can be expressed as follows: 5'-DNA-3' is expressed as N-polypeptide-C One polypeptide chain-C-loop-N-second polypeptide chain-C. According to the present invention, said loop has been cleaved in situ, so that finally the polypeptide / protein of the present invention N-first polypeptide chain-C-N-second polypeptide chain-C is obtained in a double-chain structure.
[0085] The phrase "the second chain of the protein / polypeptide has 1-20 amino acid residues in the N-terminal direction of a cysteine residue as the N-terminus and has the pentapeptide sequence...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com