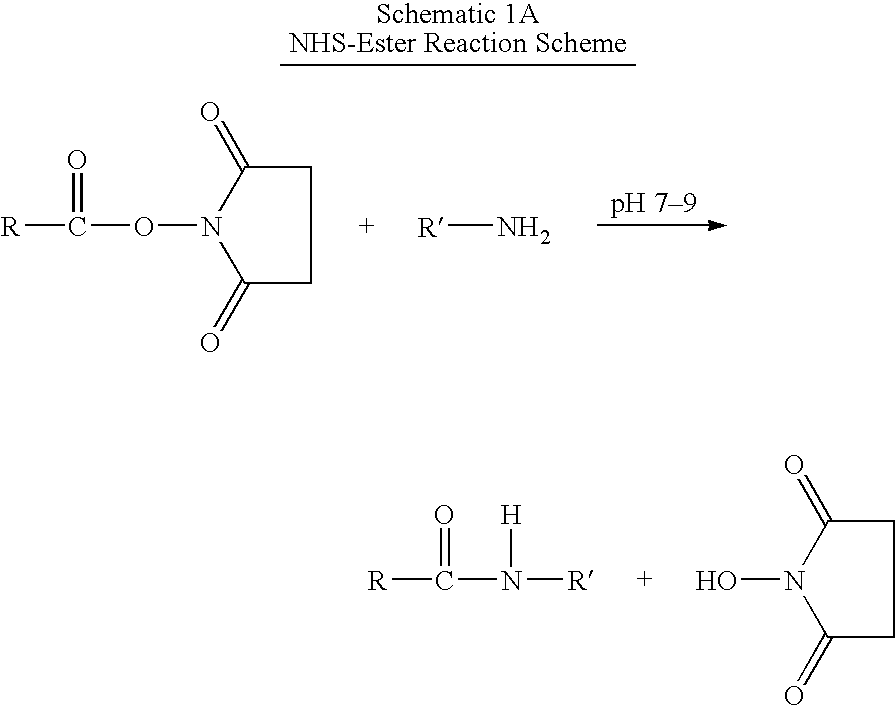
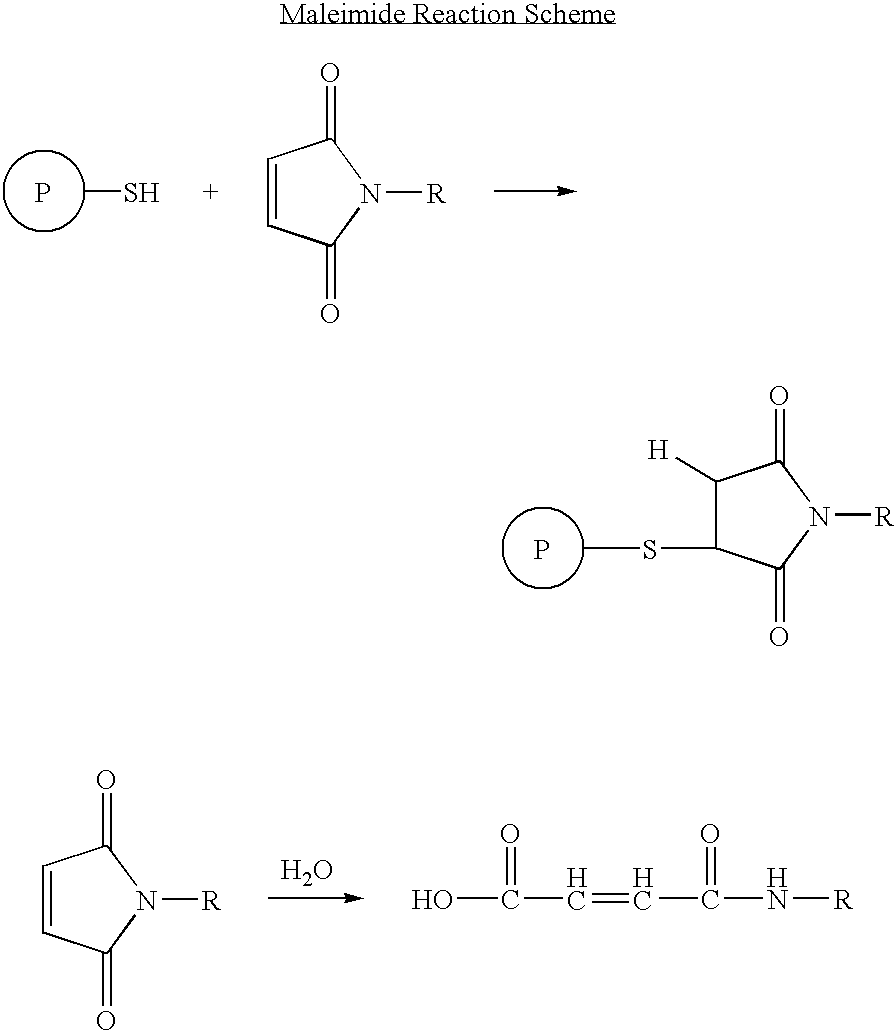
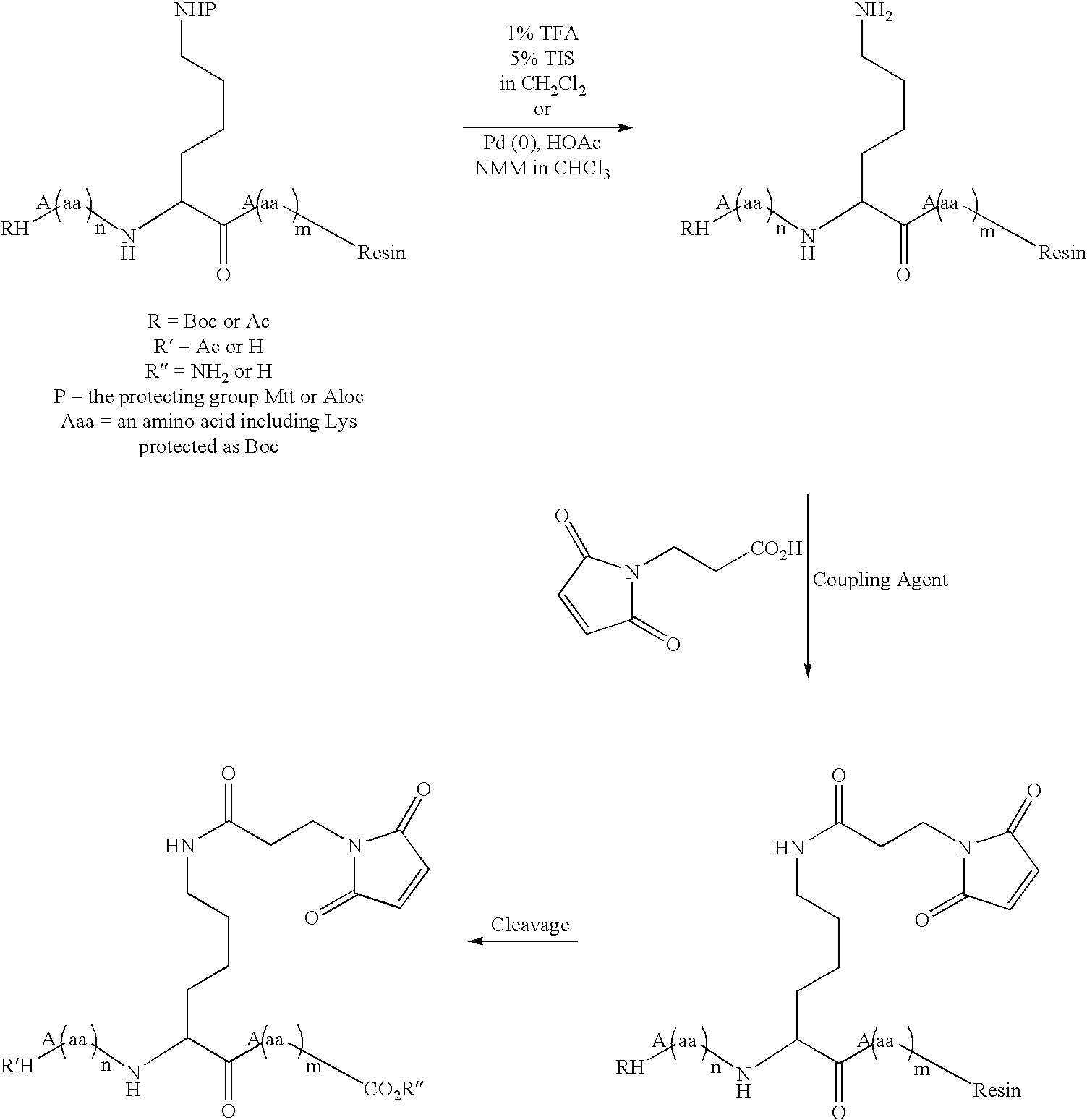
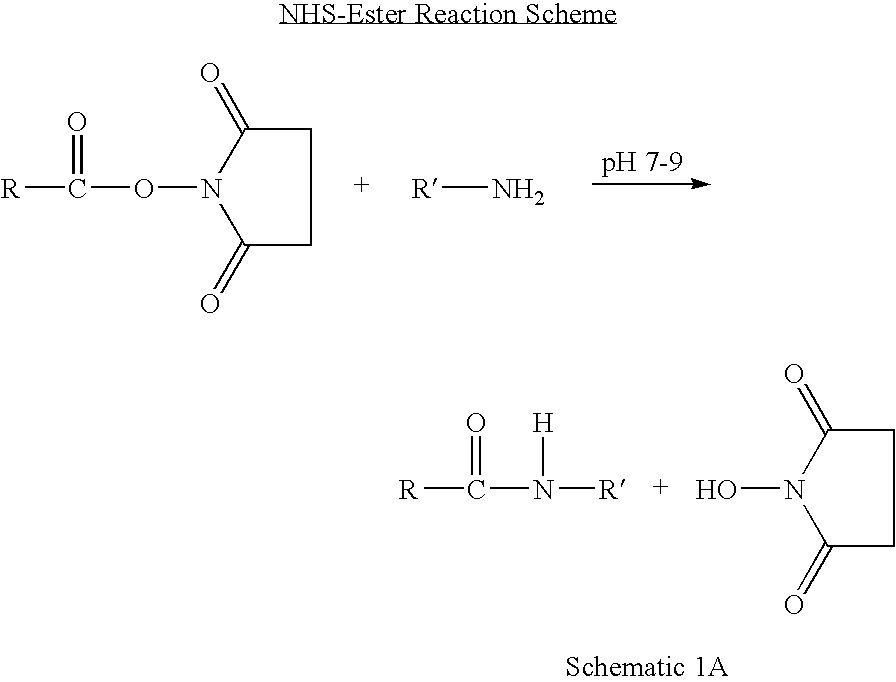
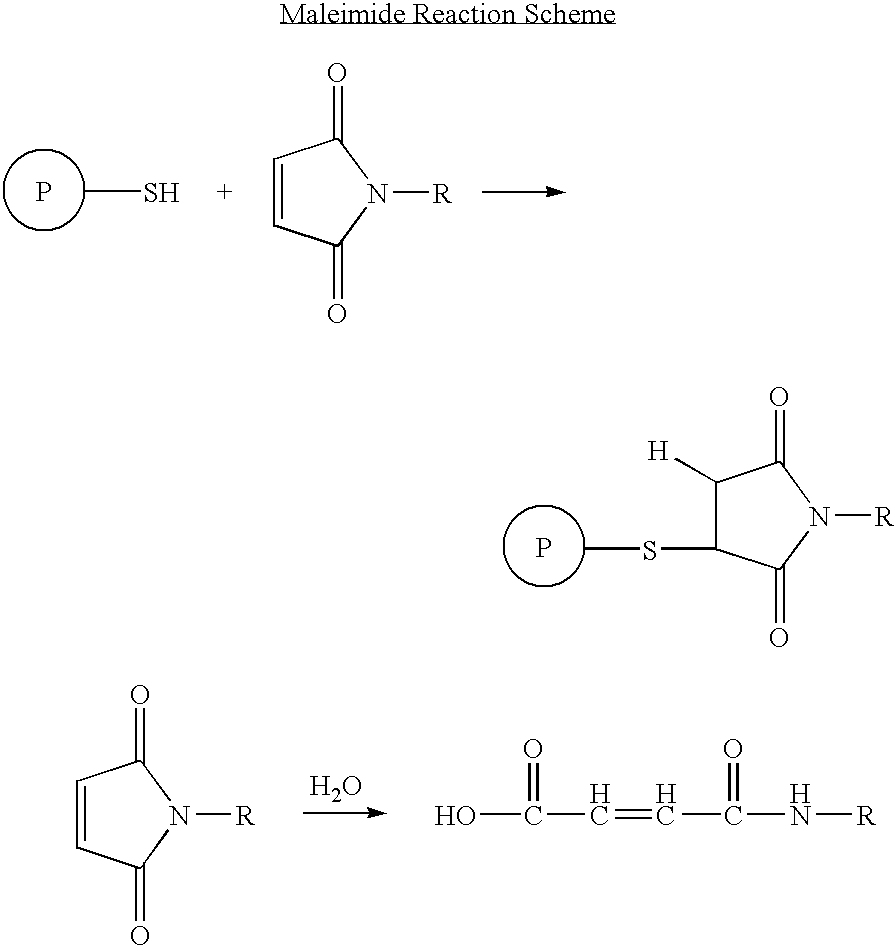
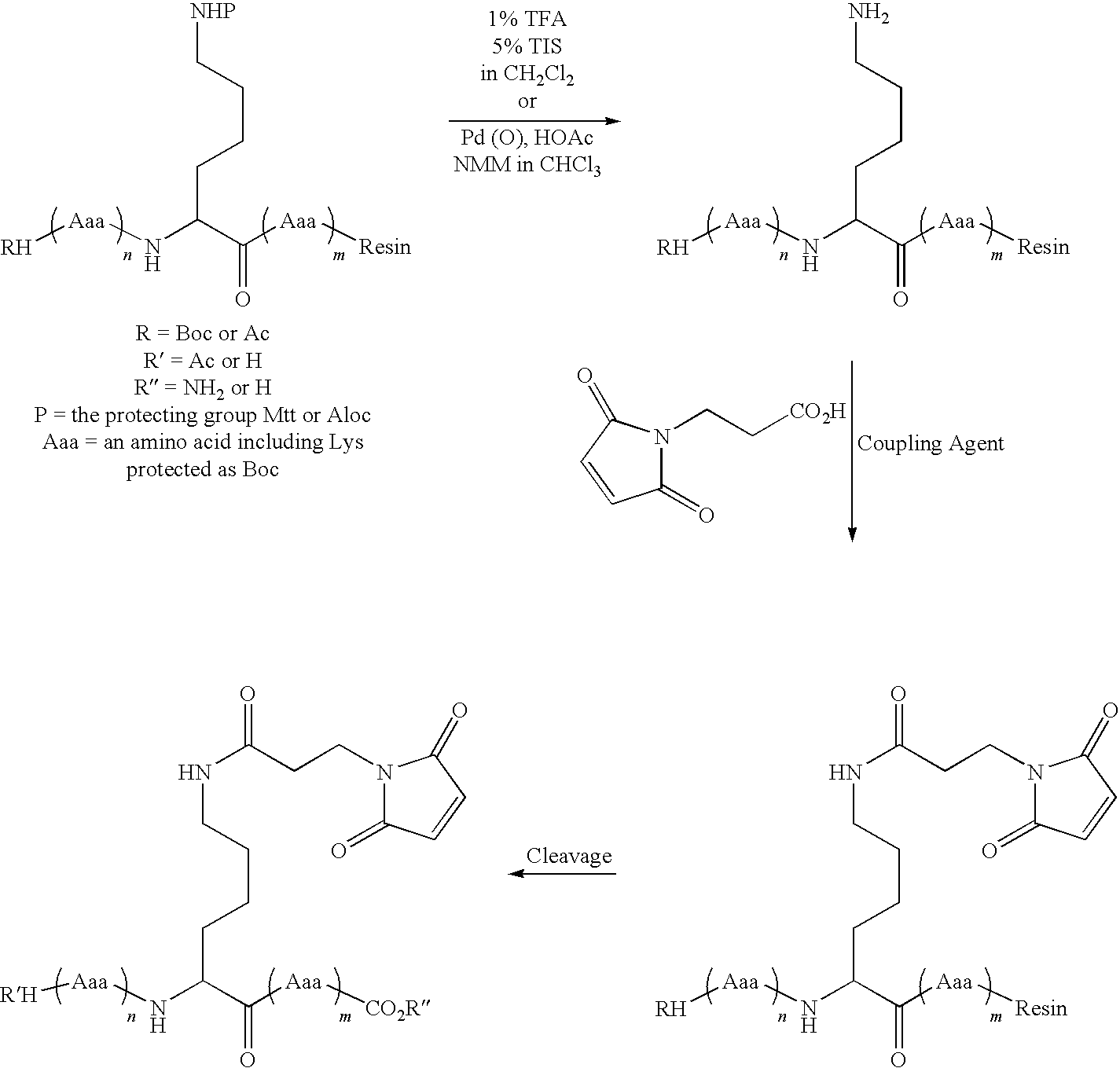
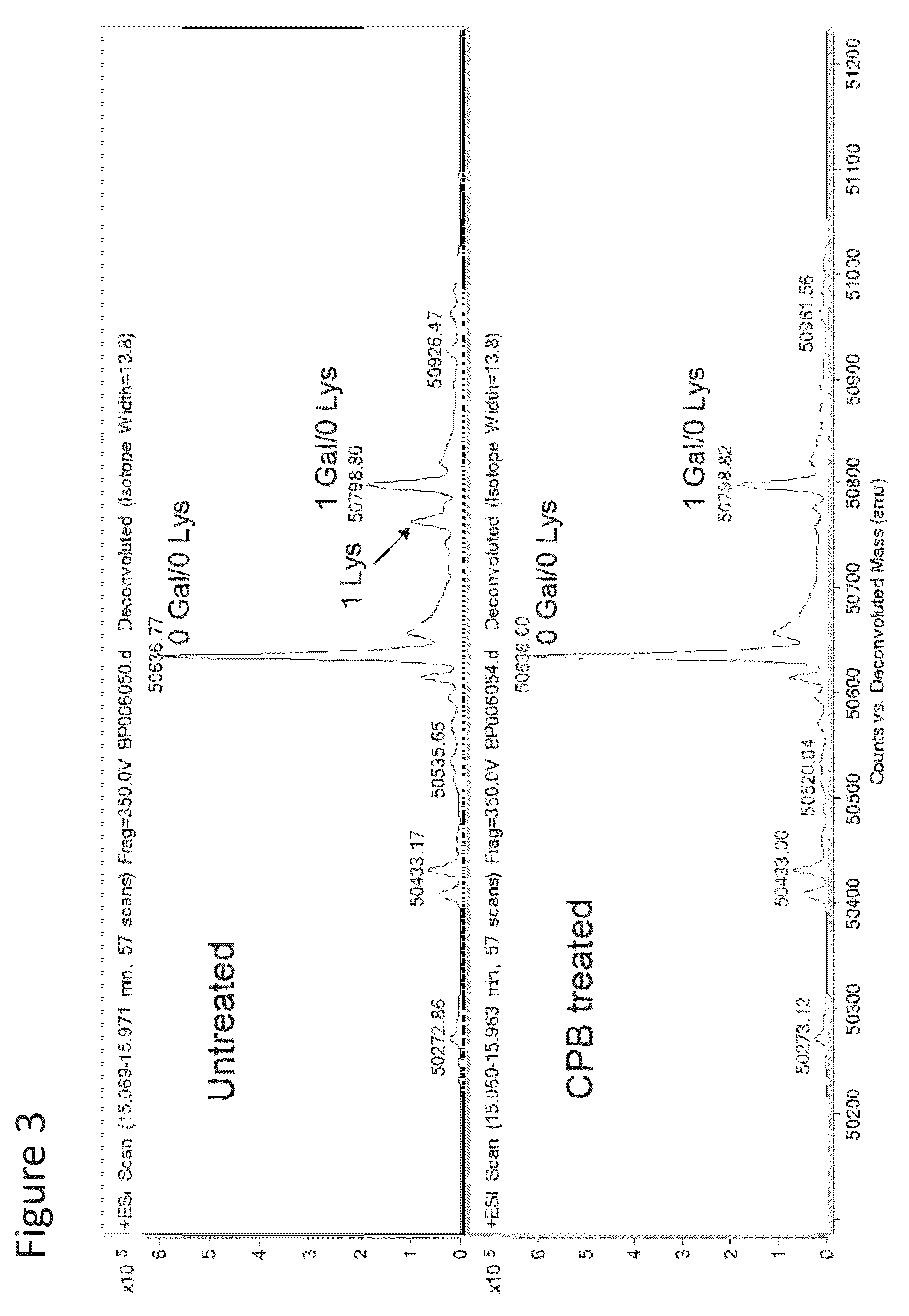
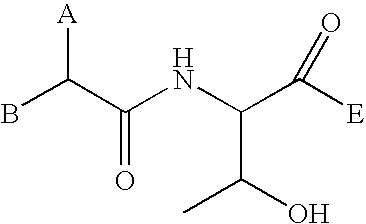
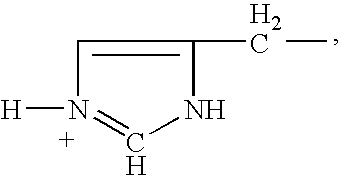
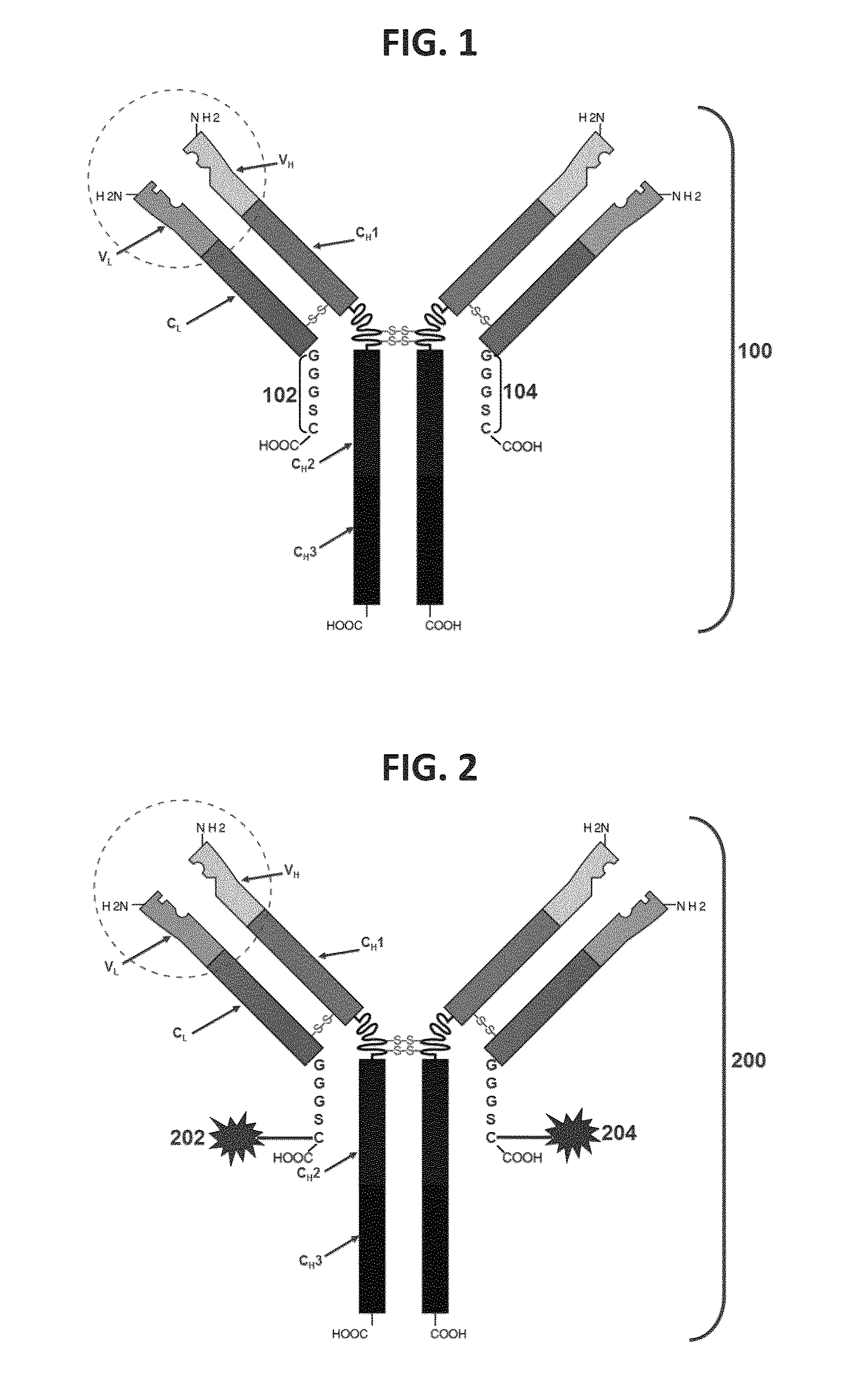
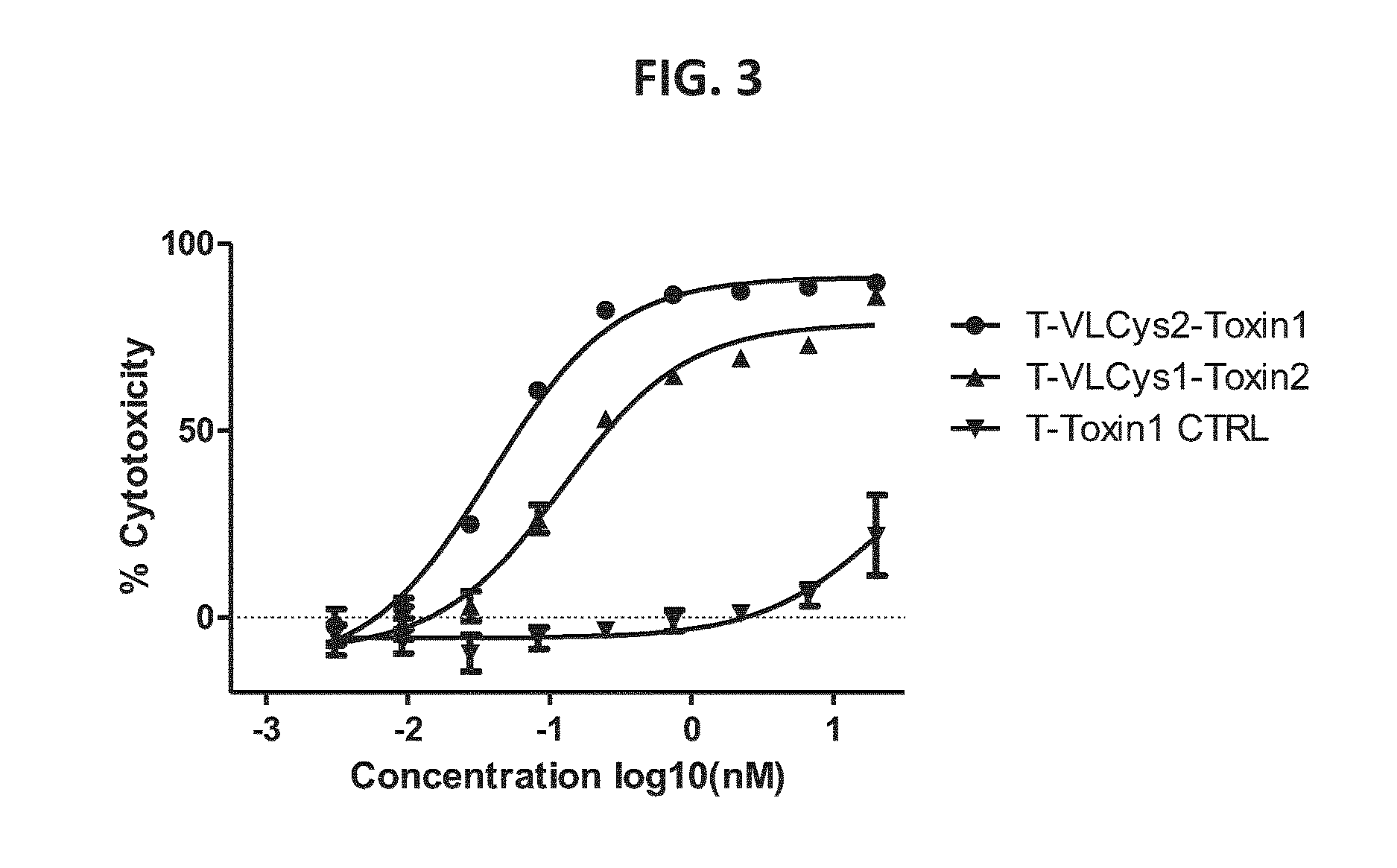
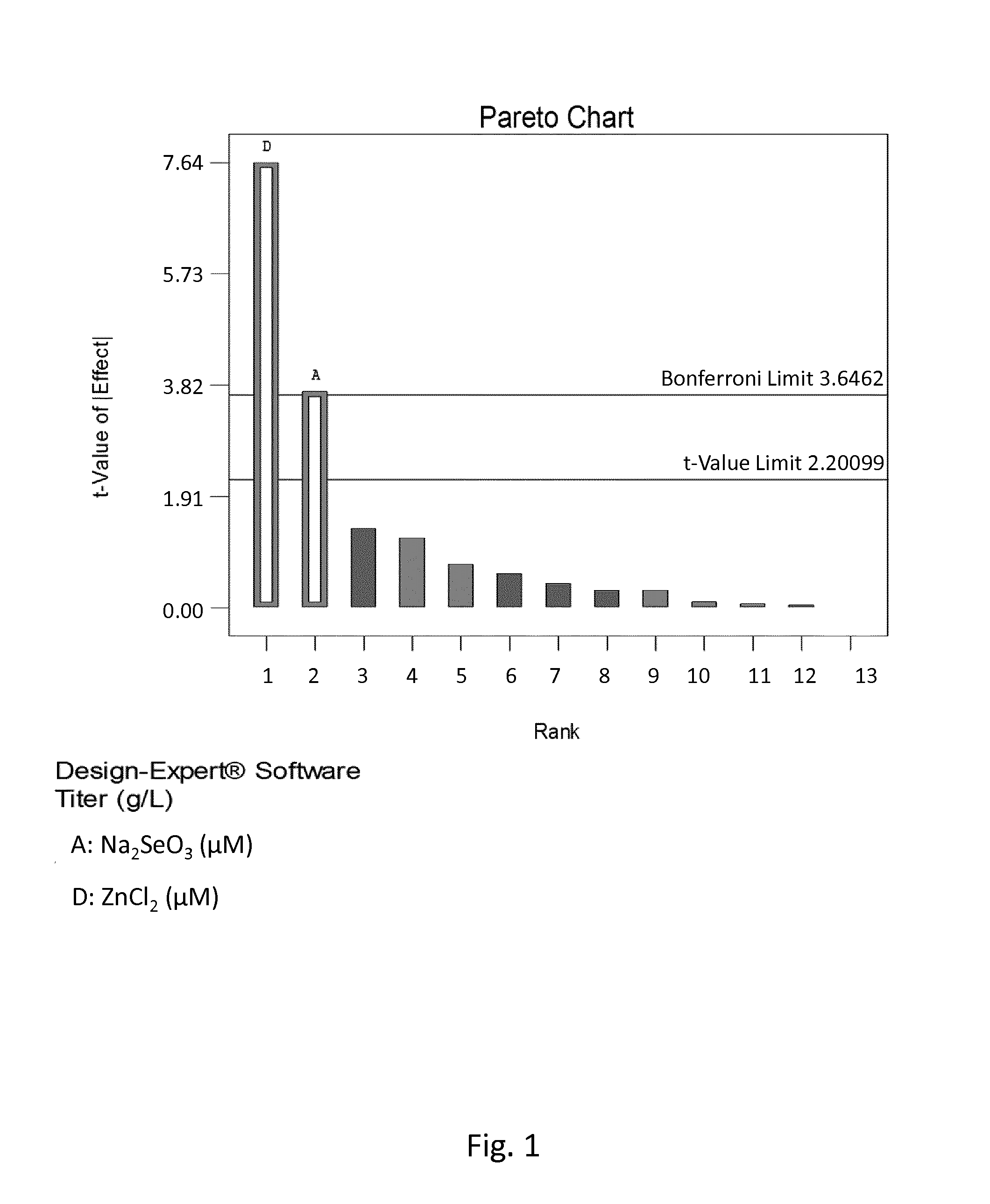
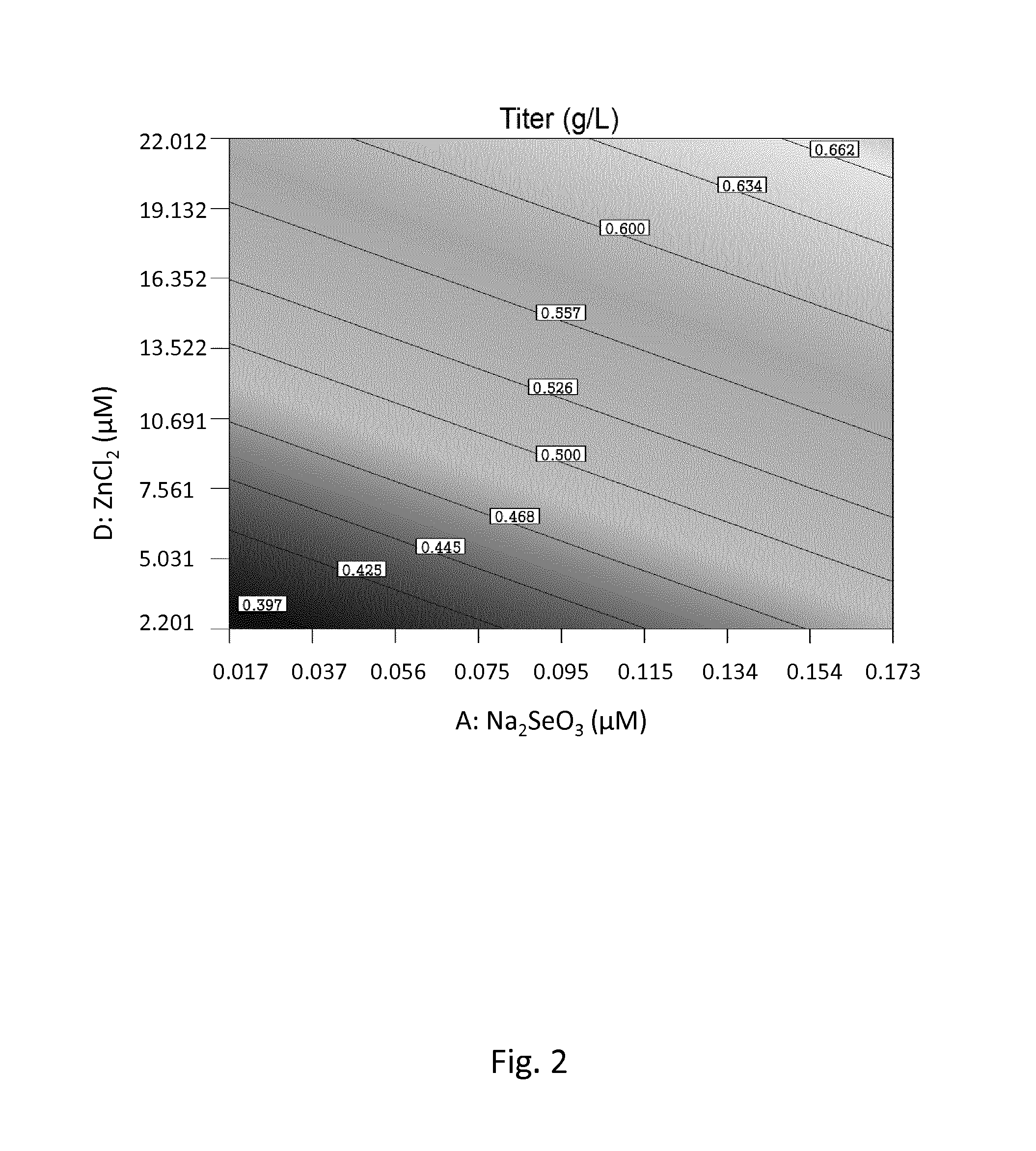
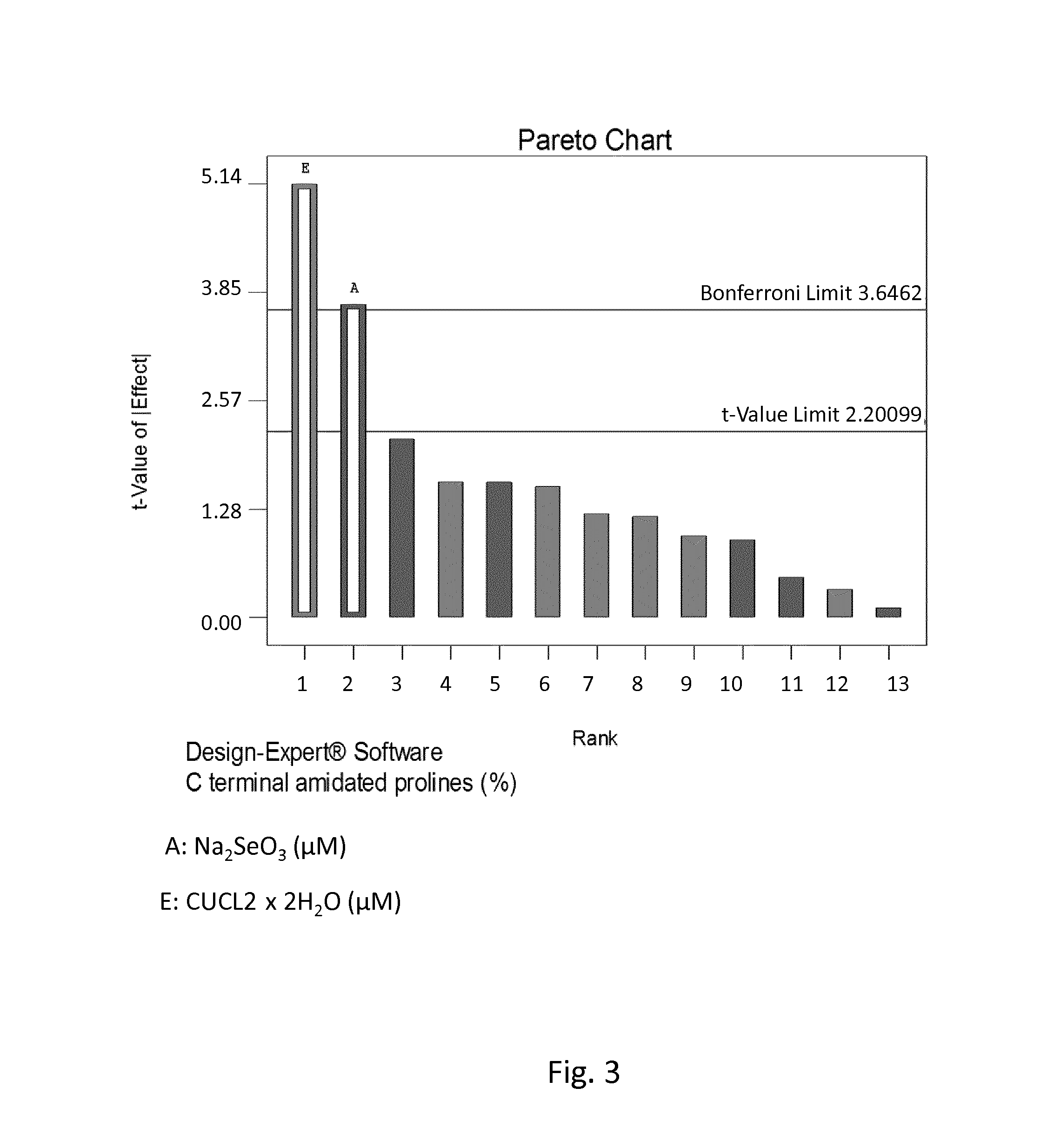
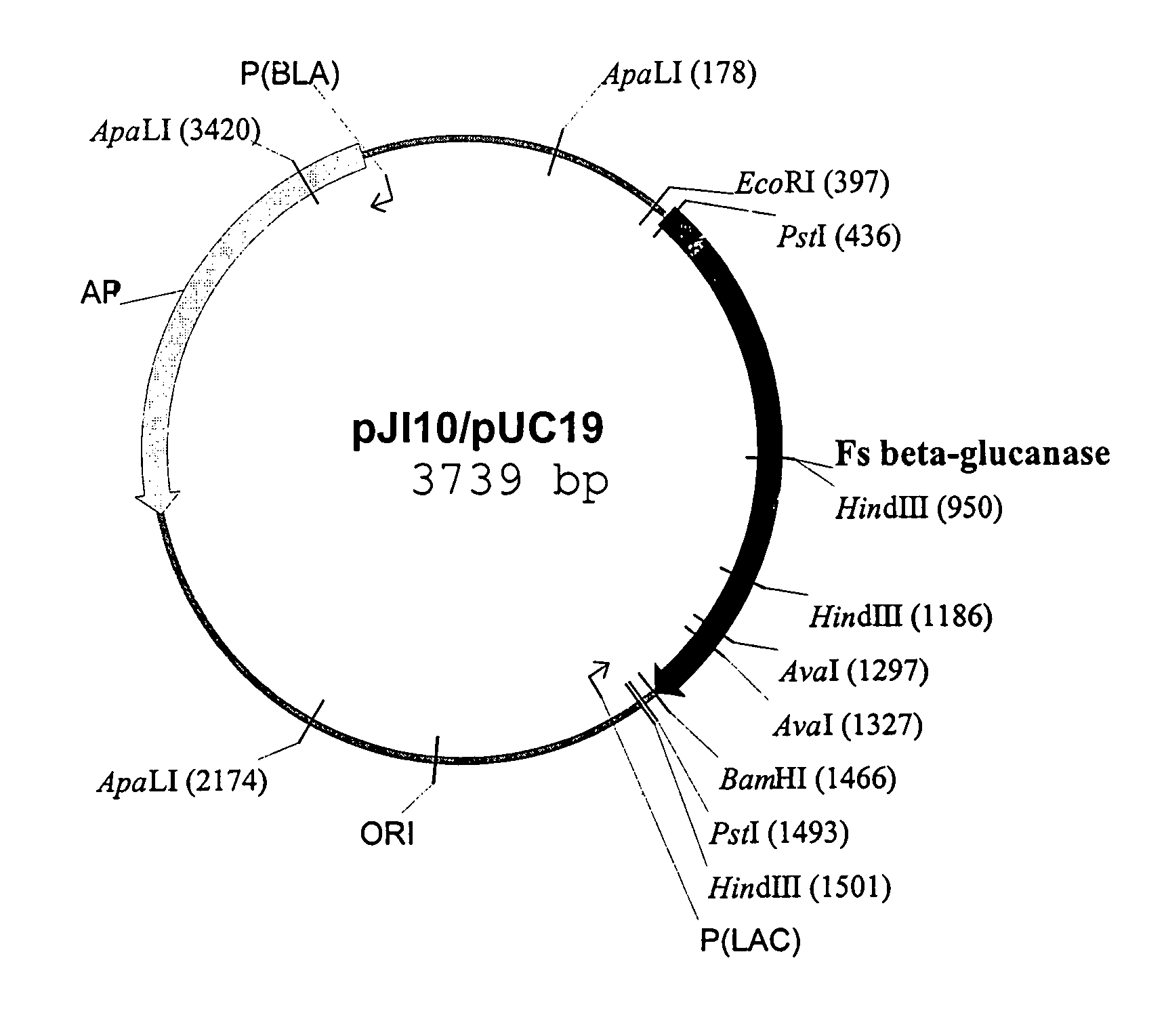
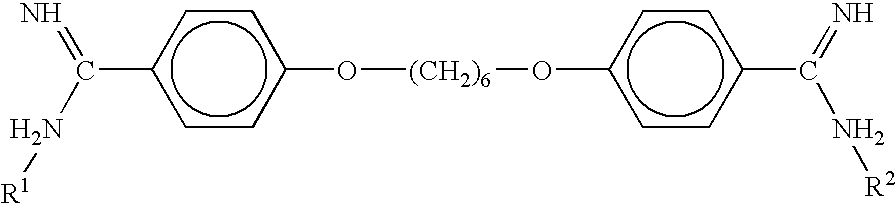Patents
Literature
88 results about "C-Terminal Amino Acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
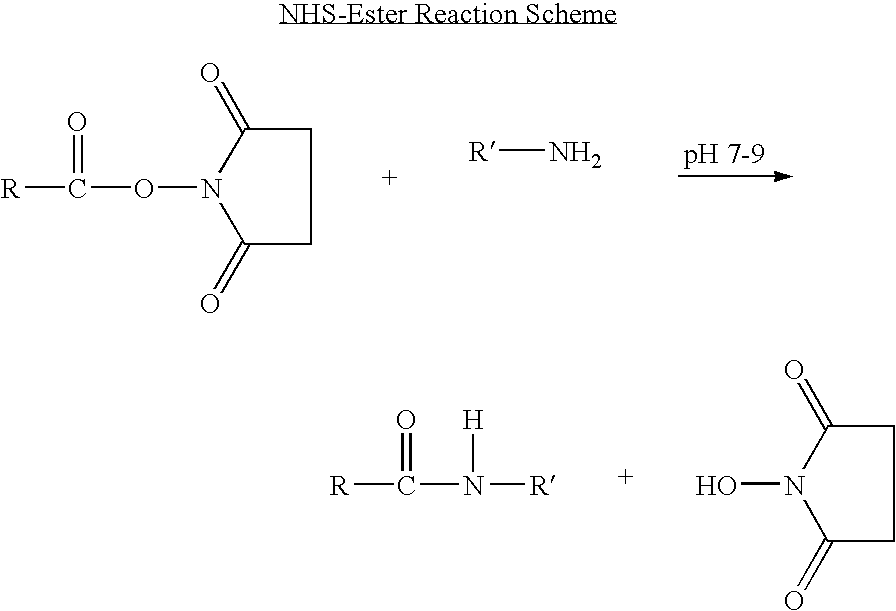
In the molecule of a peptide, the amino acid residue on one end has an amine group on the alpha carbon. This amino acid residue is called the N-terminal of the peptide. The amino acid residue on the other end has a carboxylic acid group on the alpha carbon. This amino acid is called the C-terminal.
Protection of endogenous therapeutic peptides from peptidase activity through conjugation to blood components
InactiveUS6887470B1Easy to testPeptide/protein ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsBlood componentCombinatorial chemistry
A method for protecting a peptide from peptidase activity in vivo, the peptide being composed of between 2 and 50 amino acids and having a C-terminus and an N-terminus and a C-terminus amino acid and an N-terminus amino acid is described. In the first step of the method, the peptide is modified by attaching a reactive group to the C-terminus amino acid, to the N-terminus amino acid, or to an amino acid located between the N-terminus and the C-terminus, such that the modified peptide is capable of forming a covalent bond in vivo with a reactive functionality on a blood component. In the next step, a covalent bond is formed between the reactive group and a reactive functionality on a blood component to form a peptide-blood component conjugate, thereby protecting said peptide from peptidase activity. The final step of the method involves the analyzing of the stability of the peptide-blood component conjugate to assess the protection of the peptide from peptidase activity.
Owner:CONJUCHEM
Modified therapeutic peptides with extended half-lives in vivo
A method for protecting a peptide from peptidase activity in vivo, the peptide being composed of between 2 and 50 amino acids and having a C-terminus and an N-terminus and a C-terminus amino acid and an N-terminus amino acid is described. In the first step of the method, the peptide is modified by attaching a reactive group to the C-terminus amino acid, to the N-terminus amino acid, or to an amino acid located between the N-terminus and the C-terminus, such that the modified peptide is capable of forming a covalent bond in vivo with a reactive functionality on a blood component. In the next step, a covalent bond is formed between the reactive group and a reactive functionality on a blood component to form a peptide-blood component conjugate, thereby protecting said peptide from peptidase activity. The final step of the method involves the analyzing of the stability of the peptide-blood component conjugate to assess the protection of the peptide from peptidase activity.
Owner:CONJUCHEM
Chondroitinase, process for preparing the same, and pharmaceutical composition comprising the same
InactiveUS6184023B1Avoid stickingInhibit productionBacteriaHydrolasesChondroitinase ABCConcentration gradient
A crystallizable, purified chondroitinase ABC having a molecular weight of about 100,000 dalton by the measurement of the SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and the measurement by the gel permeation chromatography method, having alanine as the N-terminal amino acid and proline as the C-terminal amino acid. A process for the purification of the crystallizable purified chondroitinase ABC comprising removing nucleic acid from an surfactant solution extract obtained from cells of chondroitinase ABC-producing microorganisms and chromatographically treating by concentration gradient elution using a weak cation exchange resin or a strong cation exchange resin. A composition comprising a chondroitinase and serum albumin, gelatin, or a nonionic surfactant.
Owner:SEIKAGAKU KOGYO CO LTD
Diagnosis and monitoring of diseases
InactiveUS20100120056A1Measured rapidly and convenientlyEasy diagnosisBiocidePeptide/protein ingredientsDiketopiperazinesIschemia
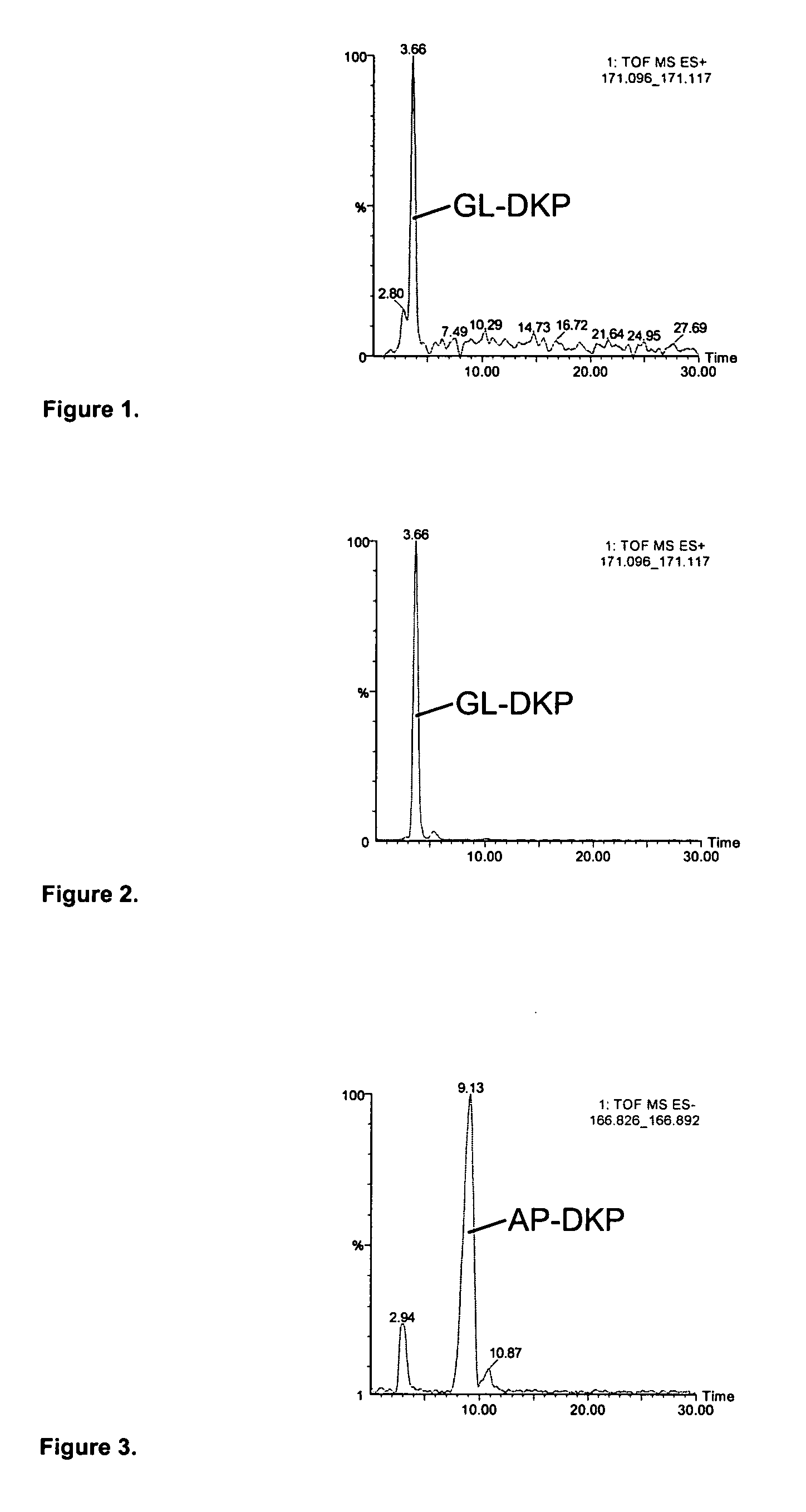
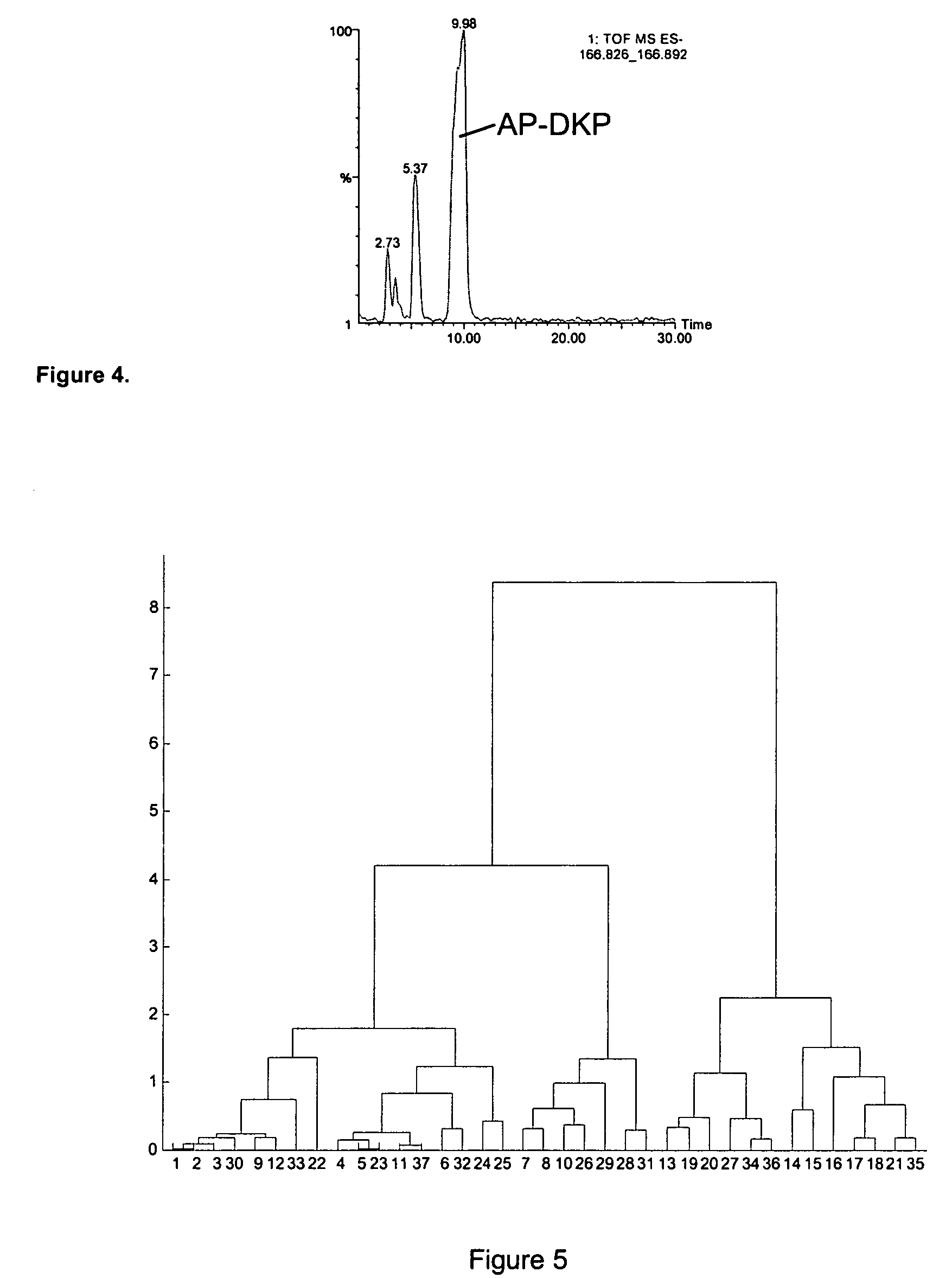
The present invention relates to the diagnosis and monitoring of diseases and conditions by quantifying markers, including degradation products of disease-associated proteins, such as diketopiperazines composed of the two N-terminal amino acids or the two C-terminal amino acids of such proteins. The methods are useful for diagnosing or monitoring various diseases, including multiple sclerosis, Alzheimer's disease and ischemia. The invention further provides binding partners specific for the markers and compositions and kits for conducting the methods of the invention.
Owner:AYTU BIOSCI
Extendin derivatives
InactiveUS7226990B2Good synergistic effectAdditional therapeutic advantageMetabolism disorderPeptide sourcesPeptideC-Terminal Amino Acid
The present invention relates to novel derivatives of exendin-4 or exendin-4 fragments, wherein the derivatives have a lipophilic substituent attached, optionally via a spacer, to an amino acid residue, which is not the N-terminal or C-terminal amino acid residue of the derivative.
Owner:NOVO NORDISK AS
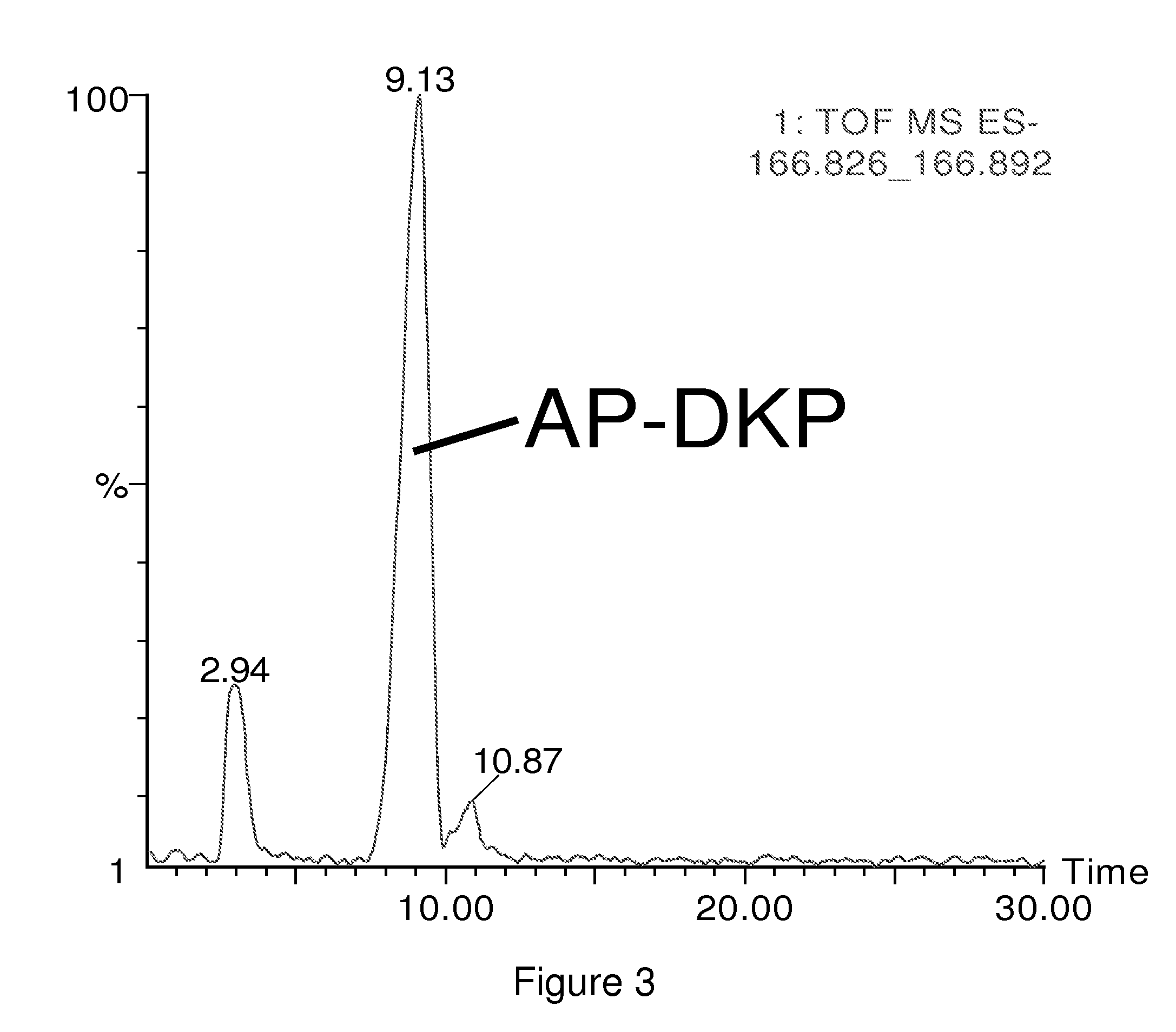
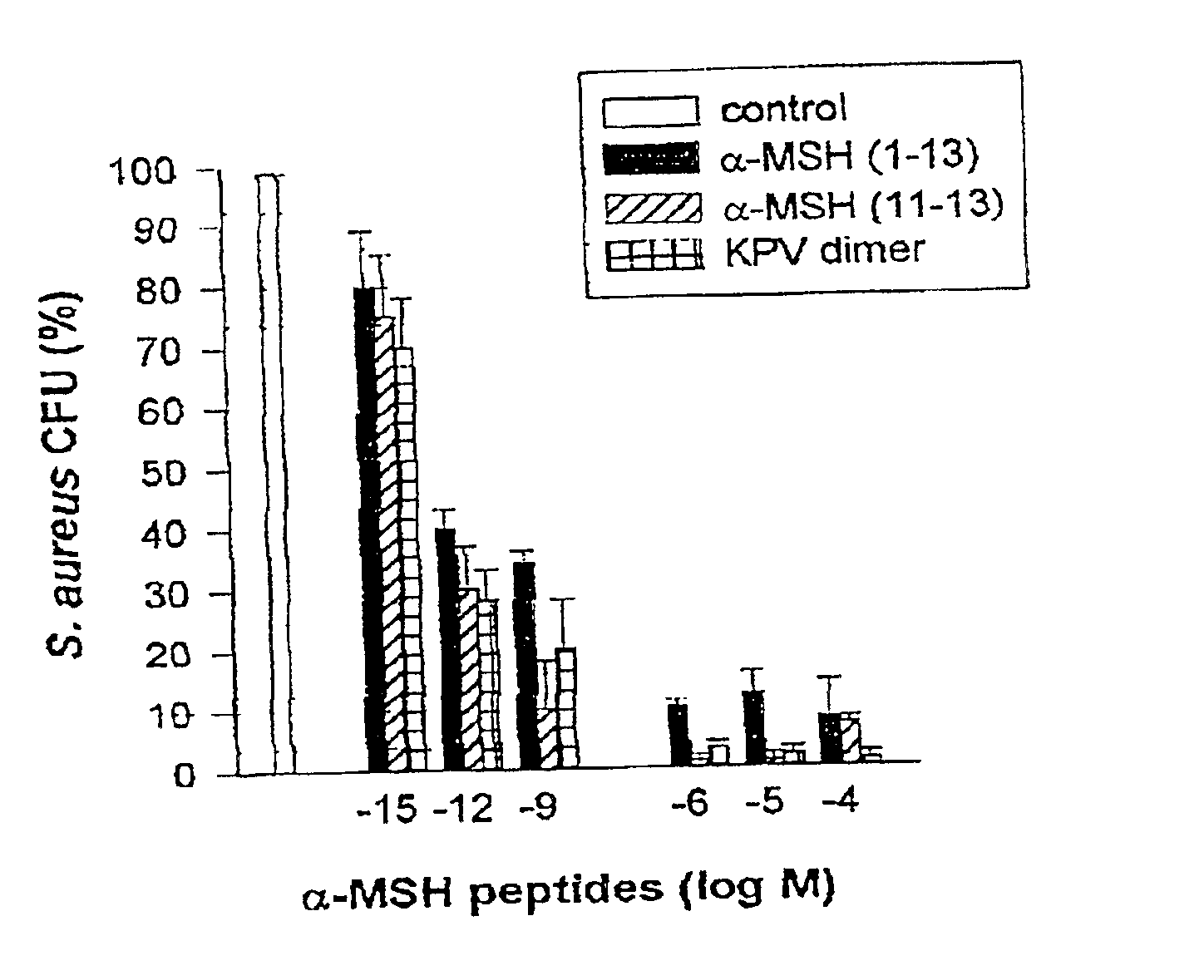
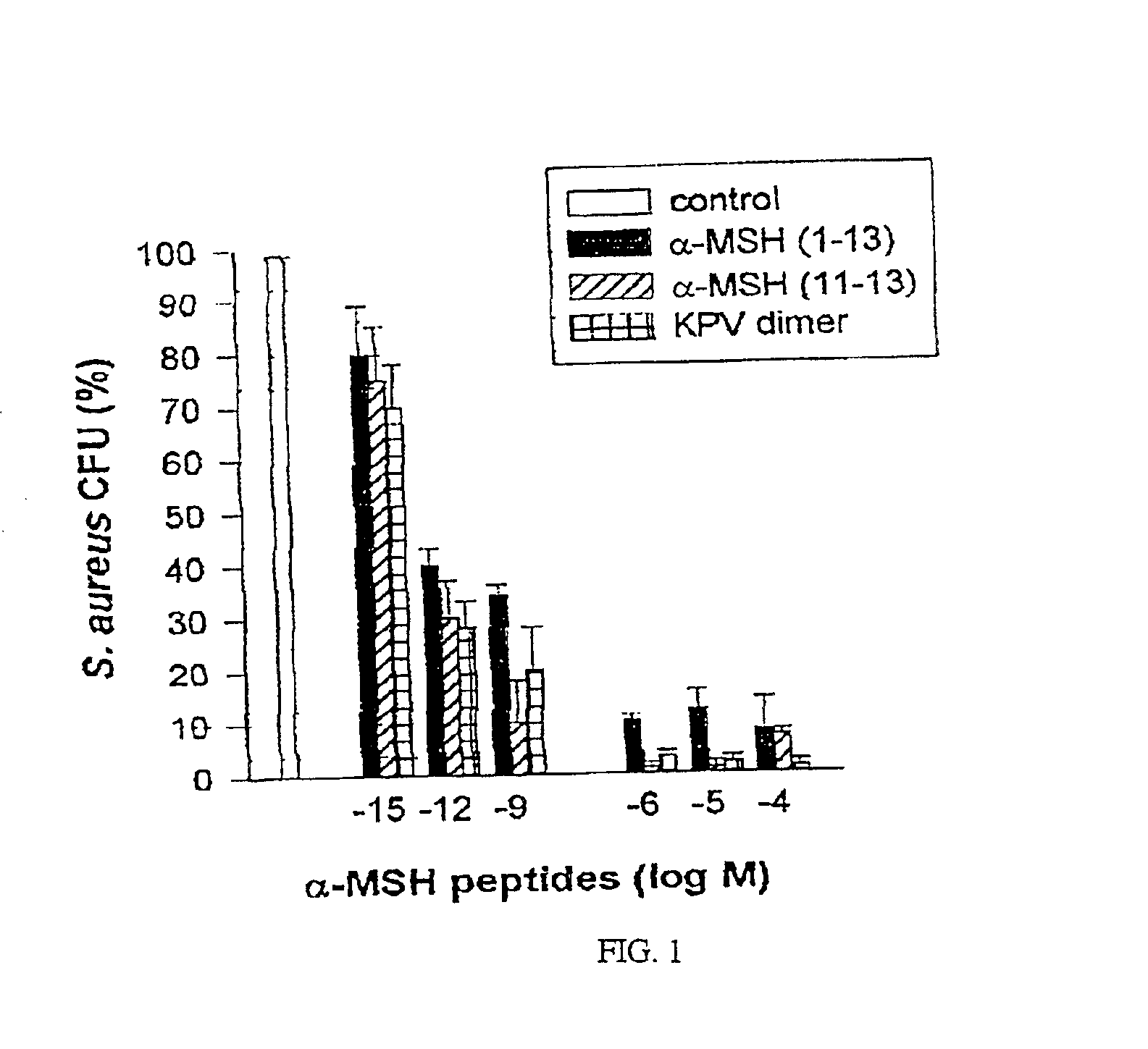
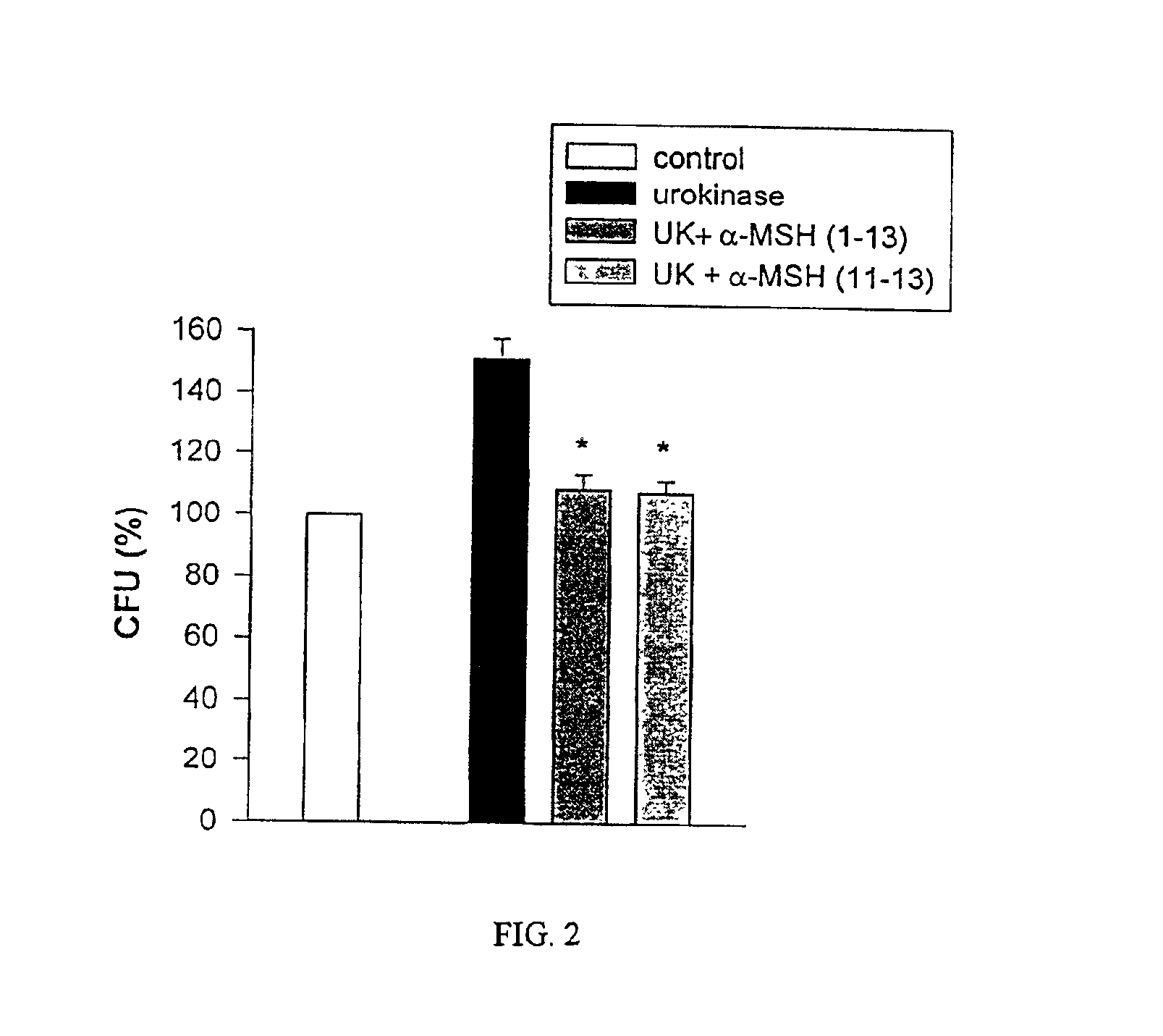
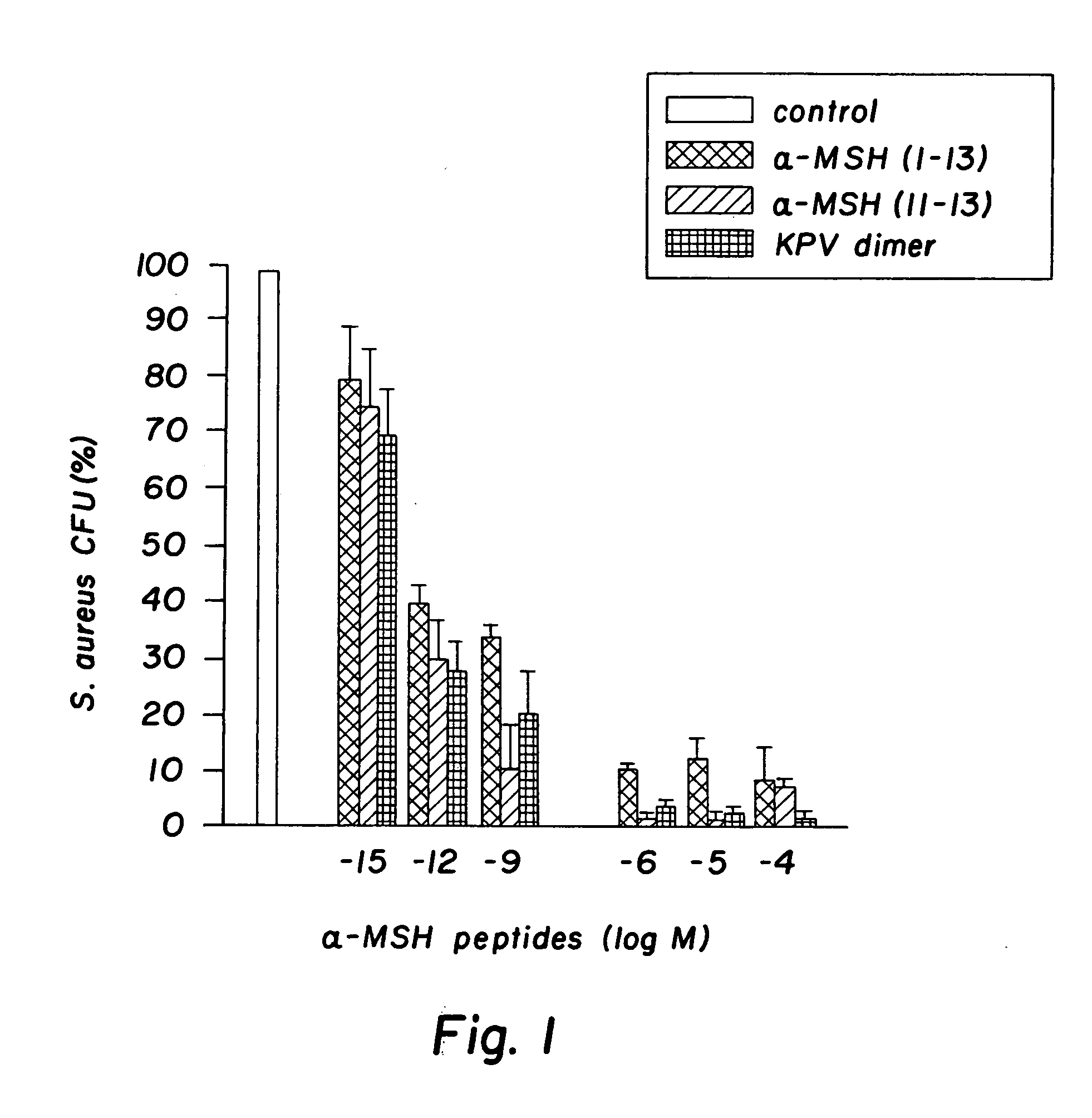
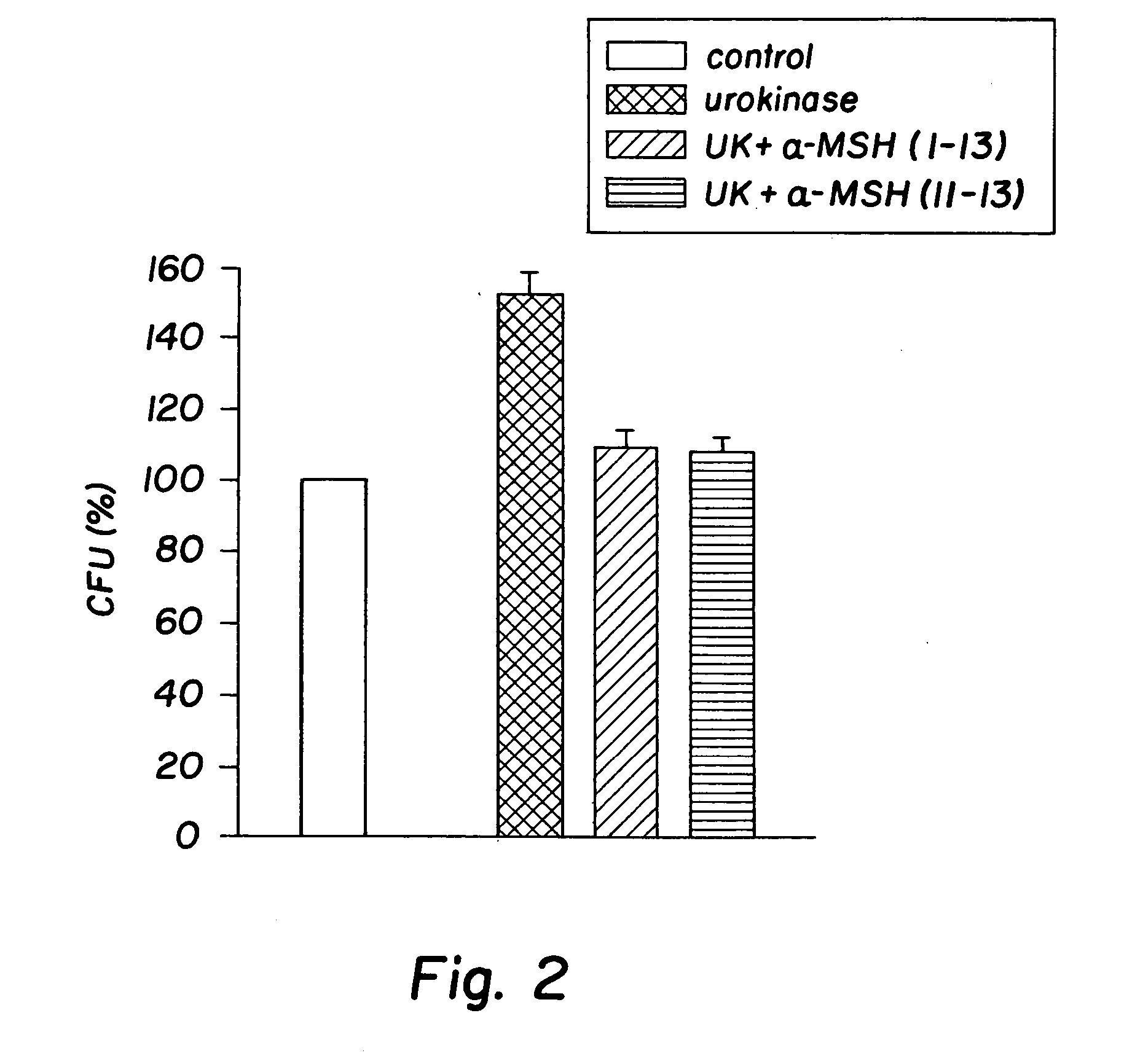
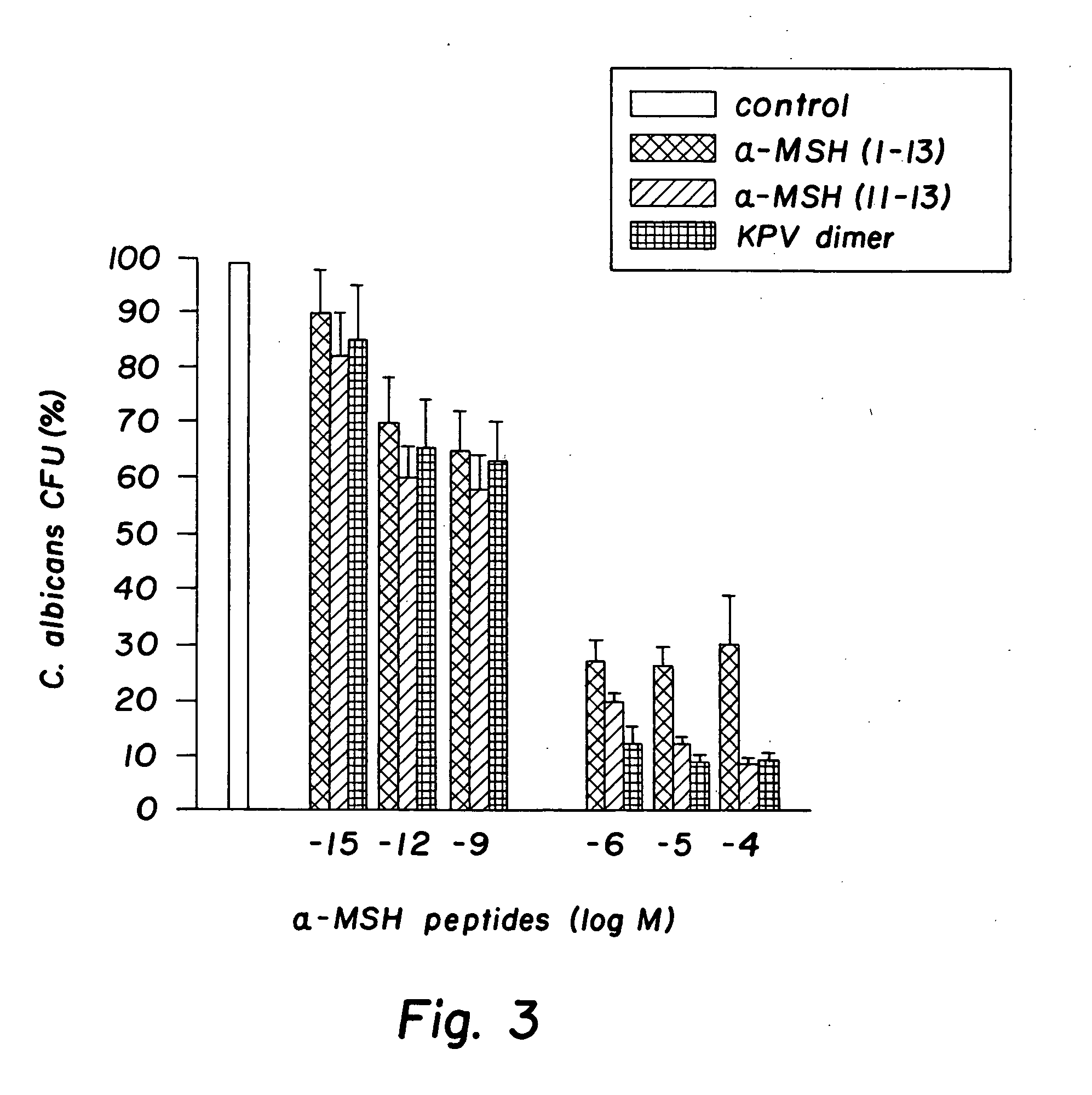
Antimicrobial amino acid sequences derived from alpha-melanocyte-stimulating hormone
Owner:MSH PHARMA INC
Peptide derivatives
A pharmacologically active peptide hormone derivative in which the parent peptide hormone has been modified by introducing either a lipophilic substituent, W, in the N-terminal amino acid or a lipophilic substituent, Z, in the C-terminal amino acid of the parent peptide hormone or an analogue thereof, said lipophilic substituent having from 8 to 40 carbon atoms, has a protracted profile of action.
Owner:NOVO NORDISK AS
Diagnosis of multiple sclerosis with diketopiperazines
InactiveUS7575929B2Measured rapidly and convenientlyEffective treatmentBiocidePeptide/protein ingredientsMedicineDiketopiperazines
The present invention relates to the diagnosis and monitoring of diseases and conditions by quantifying markers, including degradation products of disease-associated proteins, such as diketopiperazines composed of the two N-terminal amino acids or the two C-terminal amino acids of such proteins. The methods are useful for diagnosing or monitoring various diseases, including multiple sclerosis, Alzheimer's disease and ischemia. The invention further provides binding partners specific for the markers and compositions and kits for conducting the methods of the invention.
Owner:AYTU BIOSCI
Mutated anti-TNFα antibodies and methods of their use
ActiveUS8921526B2Decrease C-terminal processingDecrease in levelPeptide/protein ingredientsAntipyreticHeavy chainAmino acid supplementation
The present invention is directed to modified antibodies, including anti-TNFα antibodies, in which C-terminal amino acids of heavy chain sequences are modified from a native sequence of proline-glycine-lysine (“PGK”) to one that includes a proline positioned between the glycine and lysine, resulting in a C-terminal sequence of proline-glycine-proline-lycine (“PGPK”). The invention further provides methods of producing and using such antibodies.
Owner:ABBVIE INC
Personal care compositions
Personal care compositions comprising a dipeptide; one or more of the following: a pentapeptide, a second dipeptide, a vitamin B3 compound, a hexamindine compound, and / or a sugar amine; and a dermatologically acceptable carrier. Methods of using such compositions to treat the condition of keratinous tissue. In certain embodiments, the C terminal amino acid of said dipeptide is threonine.
Owner:THE PROCTER & GAMBLE COMPANY
Protection of exendin-4 peptides through conjugation
InactiveUS20090093408A1Easy to testPeptide/protein ingredientsSerum albuminBlood componentCombinatorial chemistry
A method for protecting a peptide from peptidase activity in vivo, the peptide being composed of between 2 and 50 amino acids and having a C-terminus and an N-terminus and a C-terminus amino acid and an N-terminus amino acid is described. In the first step of the method, the peptide is modified by attaching a react group to the C-terminus amino acid, to the N-terminus amino acid, or to an amino acid located between the N-terminus and the C-terminus, such that the modified peptide is capable of forming a covalent bond in vivo with a reactive functionality on a blood component. In the next step, a covalent bond is formed between the reactive group and a reactive functionality on a blood component to form a peptide-blood component conjugate, thereby protecting said peptide from peptidase activity. The final step of the method involves the analyzing of the stability of the peptide-blood component conjugate to assess the protection of the peptide from peptidase activity.
Owner:CONJUCHEM +1
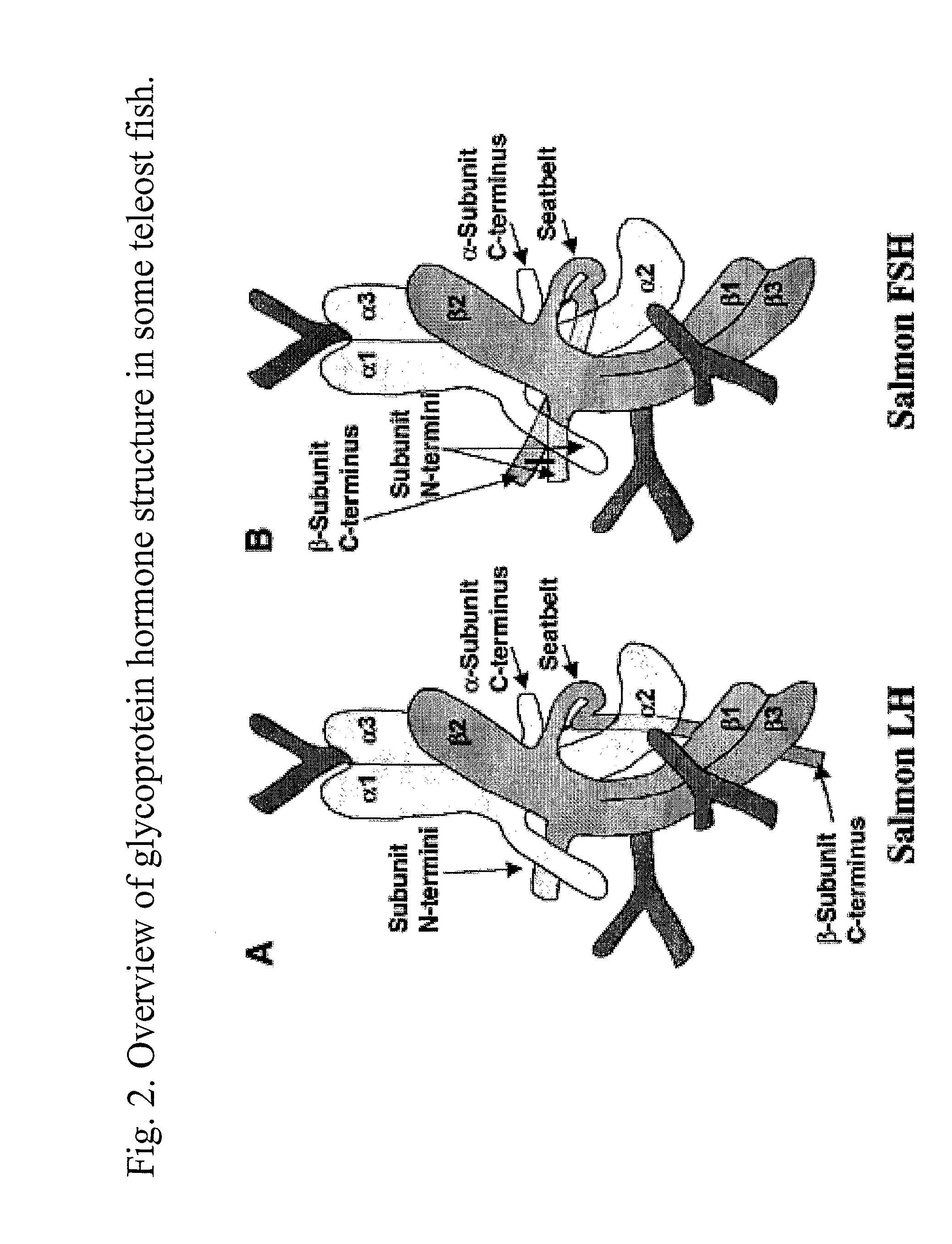
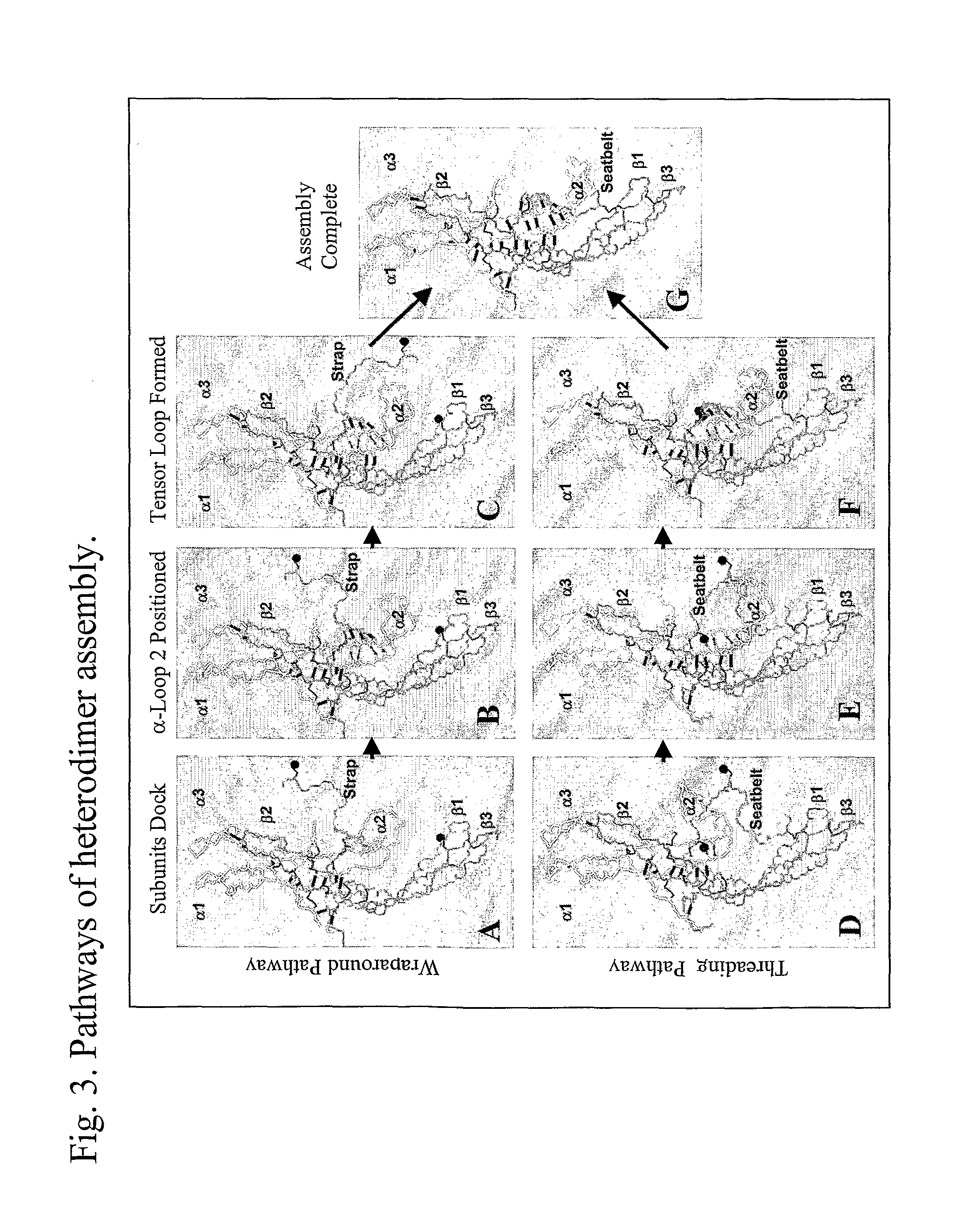
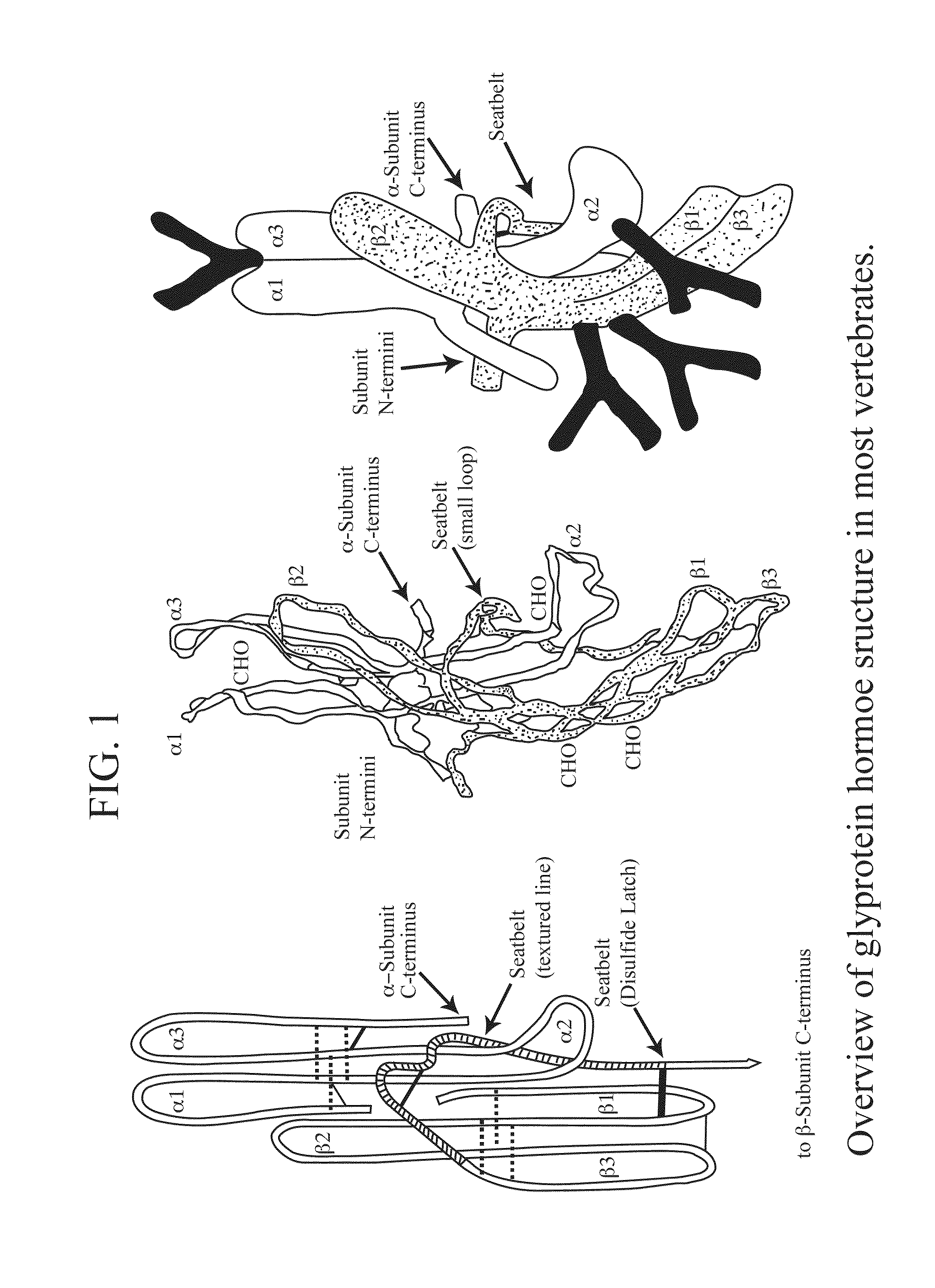
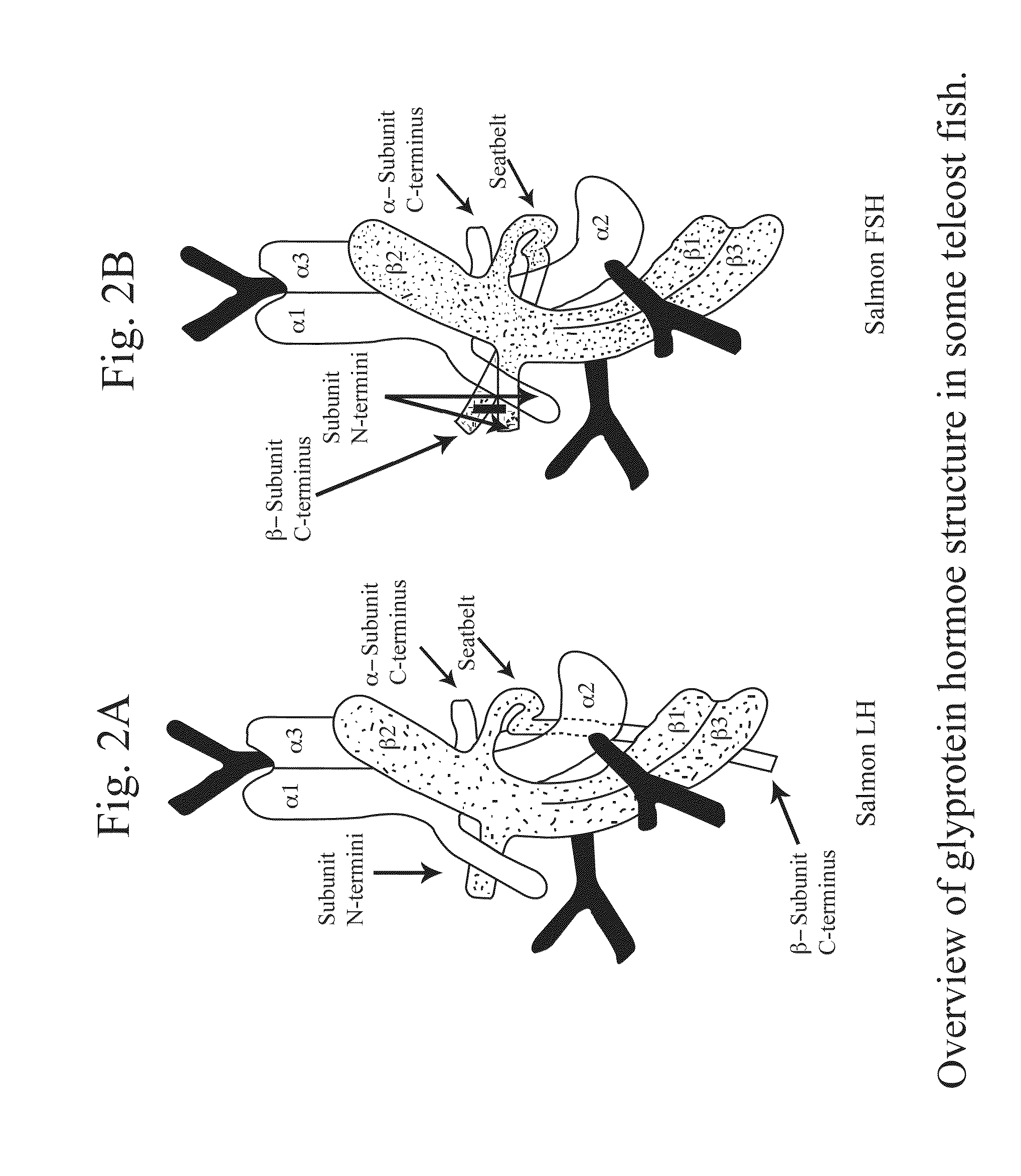
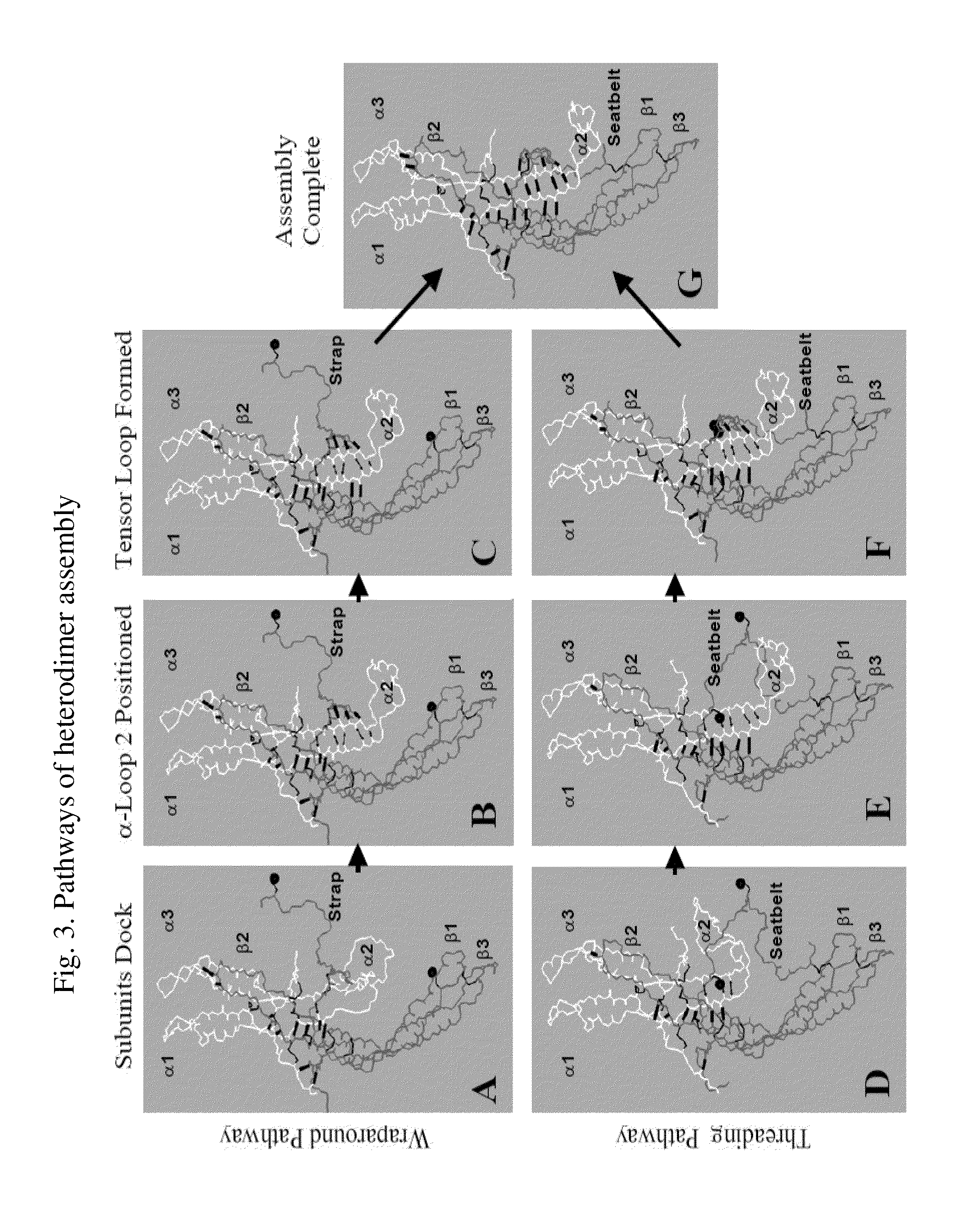
Glycoprotein Hormone Analogs
InactiveUS20090209453A1Sugar derivativesPeptide/protein ingredientsHormone analogHormones regulation
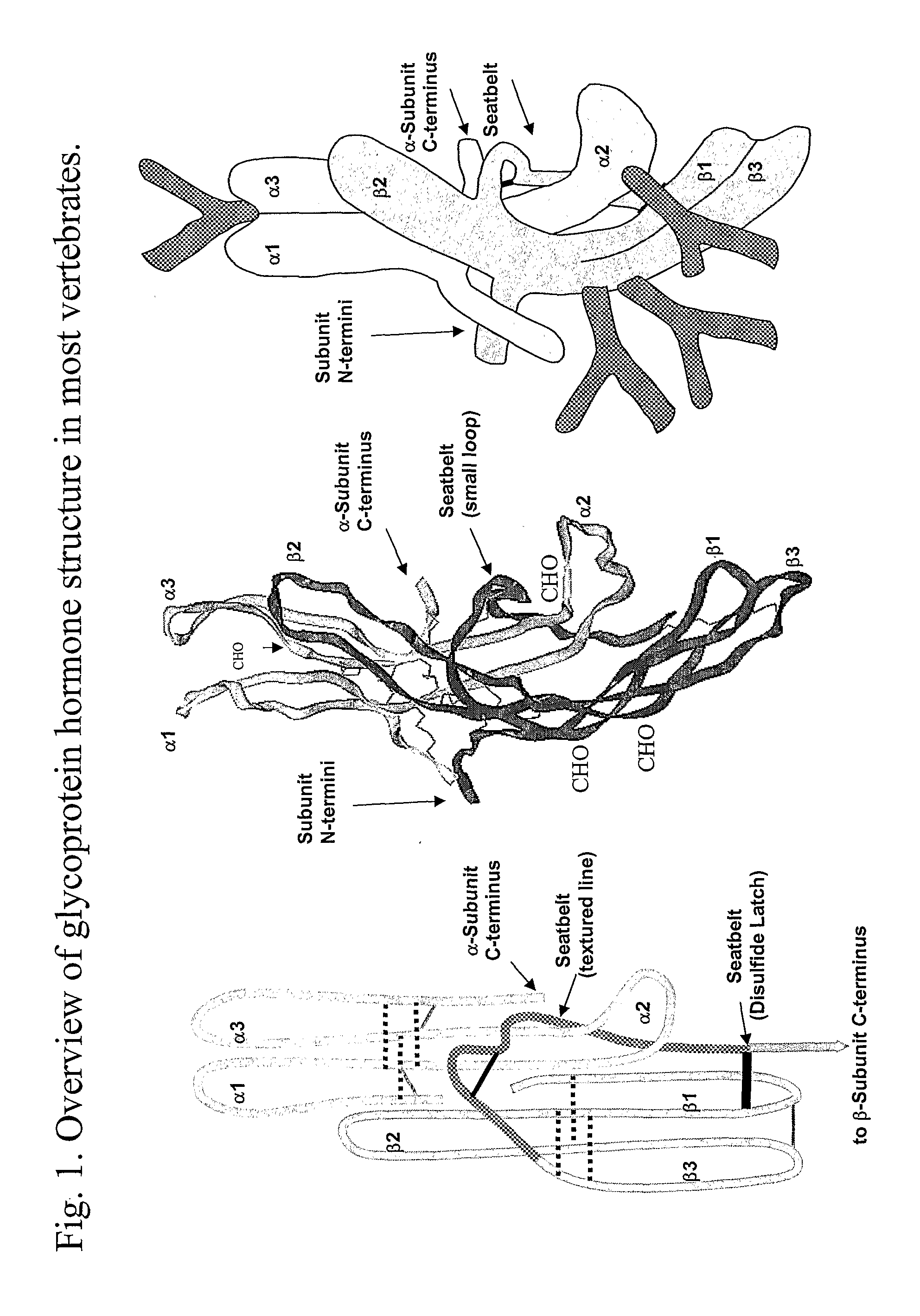
This invention relates to the field of glycoprotein hormone analogs and their uses as agonists, antagonists, targeting vectors, and immunogens. In particular, this invention describes a method for stabilizing a heterodimer that permits the preparation of functional glycoprotein hormone analogs. The analogs of present invention comprise at least one alpha subunit polypeptide and at least one beta subunit polypeptide, wherein the seatbelt region of the beta subunit is linked to the alpha subunit. The invention also provides for a beta subunit polypeptide wherein the C-terminal amino acid is from residue 10 to residue 20 of the seatbelt region.
Owner:UNIV OF MEDICINE & DENTISTRY OF NEW JERSEY
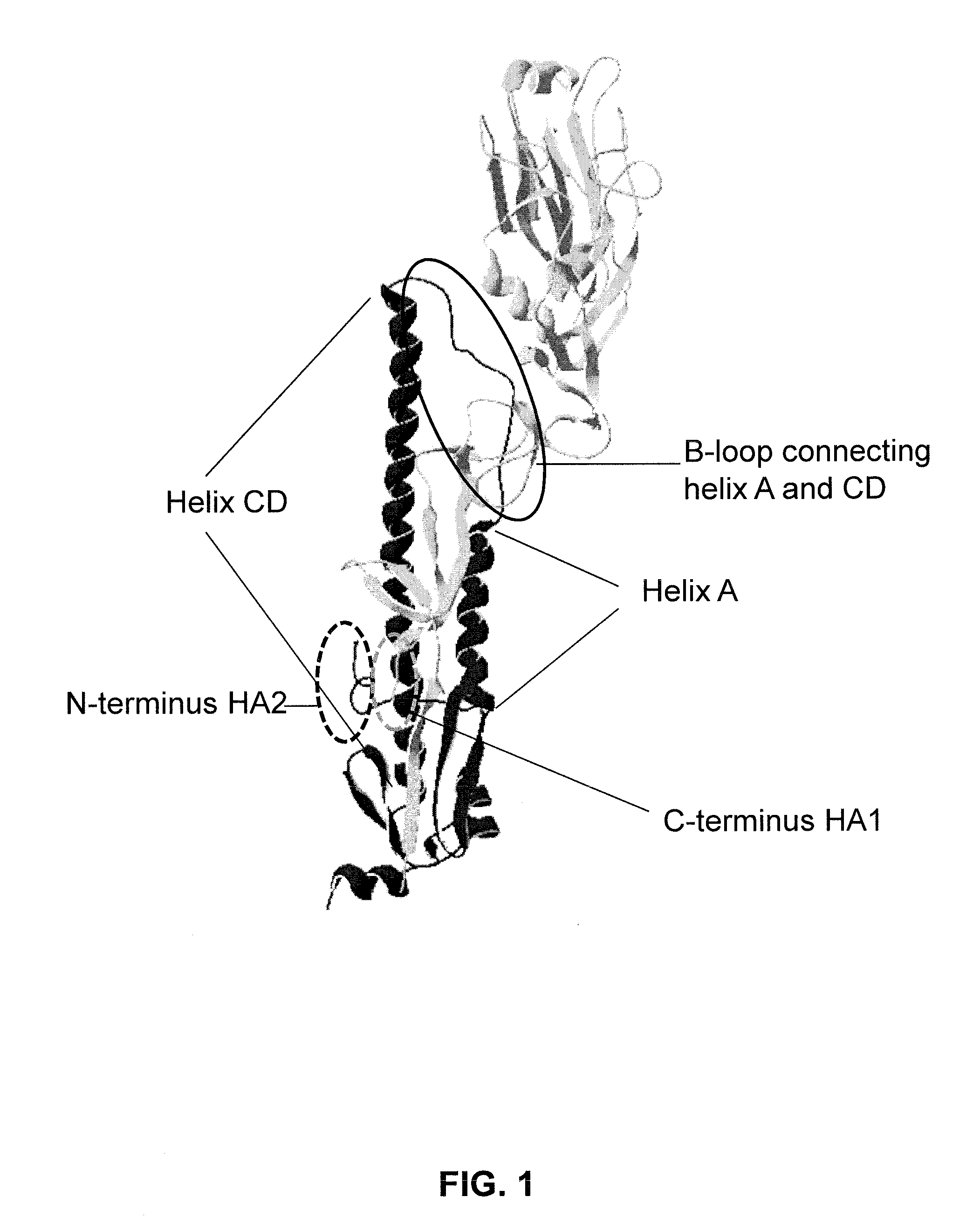
Influenza virus vaccines and uses thereof
ActiveUS20160136262A1SsRNA viruses negative-senseAntibody mimetics/scaffoldsHemagglutininInfluenza virus vaccine
Provided are influenza hemagglutinin stem domain polypeptides comprising (a) an influenza hemagglutinin HA1 domain that comprises an HA1 N-terminal stem segment comprising the amino acids from position 1 to position x, preferably from position p to position x, of the HA1 domain, covalently linked by a linking sequence of 0-50 amino acid residues to an HA1 C-terminal stem segment, comprising the amino acids from position y to and including the C-terminal amino acid of the HA1 domain; and (b) an influenza hemagglutinin HA2 domain, wherein the hemagglutinin stem domain polypeptide is resistant to protease cleavage at the junction between HA1 and HA2, and wherein one or more amino acid of the amino acids at positions 337, 340, 352, 353, 402, 406, 409, 413 and / or 416 have been mutated, as compared to the corresponding positions in wild-type influenza HA.
Owner:JANSSEN VACCINES & PREVENTION BV
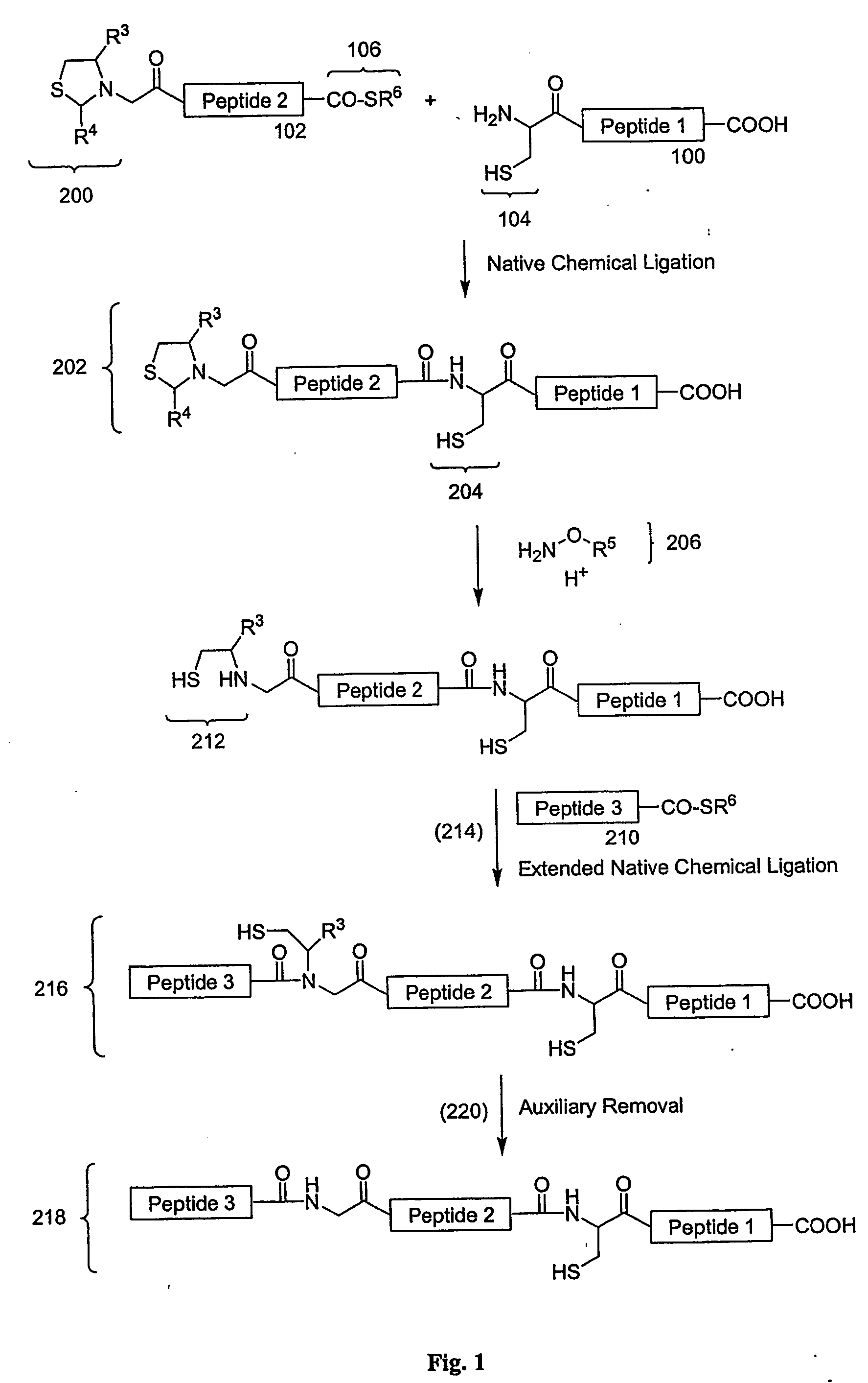
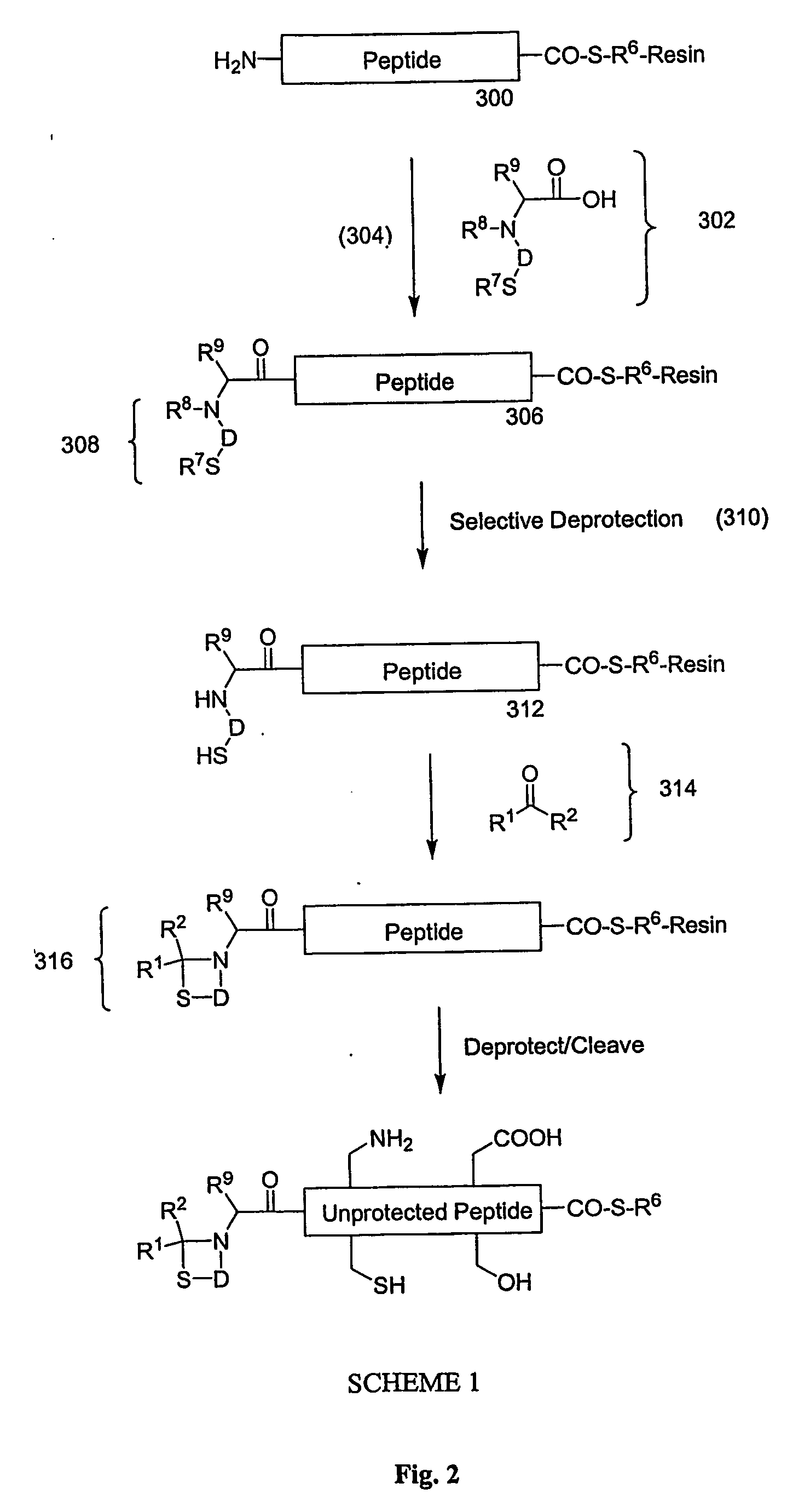
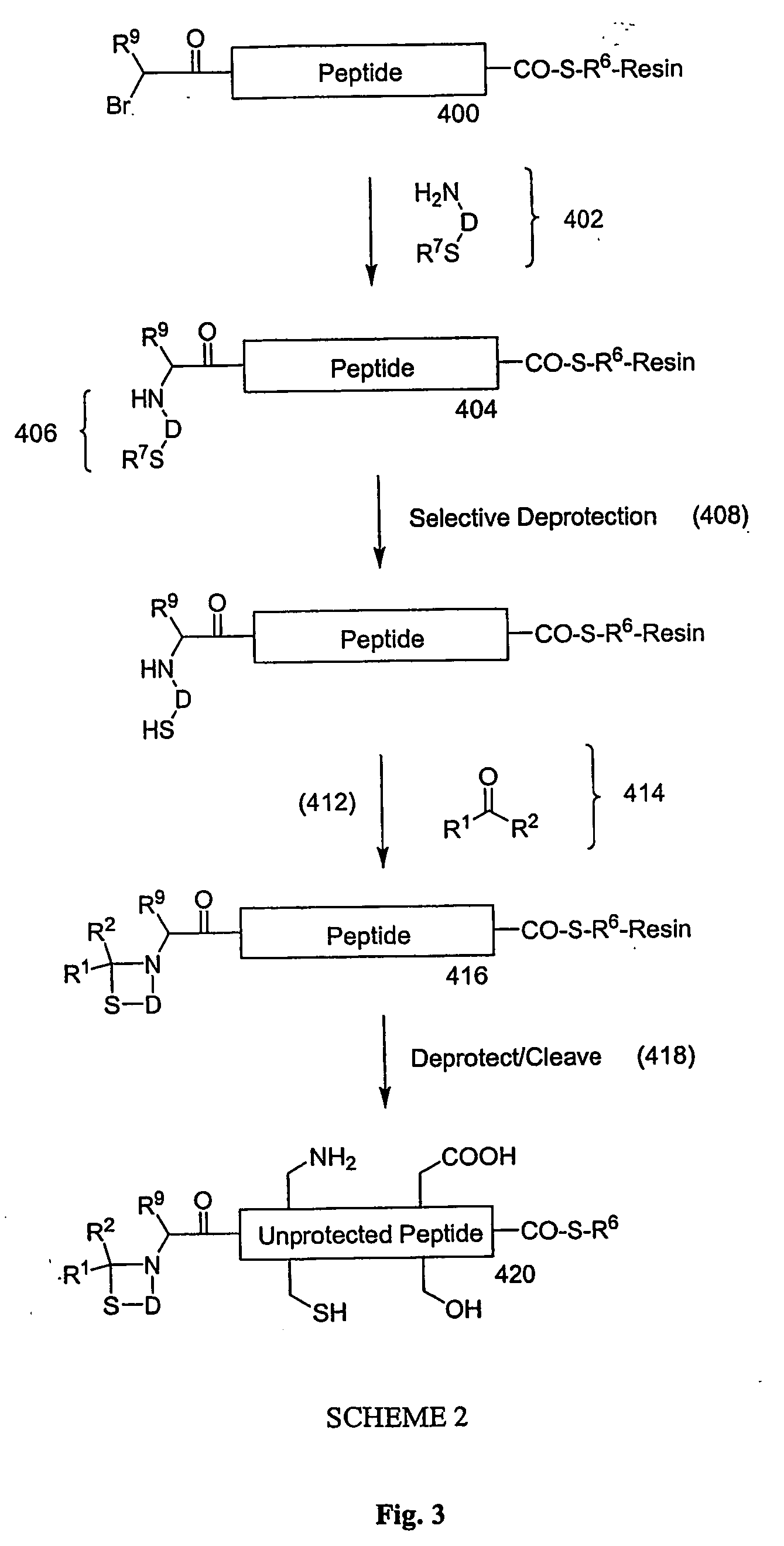
Carboxy protection strategies for acidic c-terminal amino acids in chemical ligation of oligopeptides
InactiveUS20050261473A1Avoid rearrangementPeptide-nucleic acidsPeptide/protein ingredientsThioester synthesisSide chain
The present invention provides methods of assembling oligopeptides or polypeptides in a native chemical ligation reaction that eliminates the formation of unwanted side products resulting from the presence of an unprotecte acidic C-terminal oligopeptide thioester. An important aspect of the present invention is providing side chain protected acidic C-terminal oligopeptide thioesters. The present invention is useful in methods for chemical synthesis of oligopeptides, polypeptides and proteins and improves the efficiency of native chemical ligation reactions, particularly where aspartyl or glutamyl peptide fragments are used to assemble an oligopeptide, polypeptide or protein product
Owner:ASTRAZENECA PHARMA LP
Insulin derivatives
InactiveUS20040067874A1Peptide/protein ingredientsMetabolism disorderAmino acidC-Terminal Amino Acid
The present invention relates to insulin derivatives in which a lipophilic group having from 12 to 40 carbon atoms is attached to the alpha-amino group of the N-terminal amino acid in the B-chain or to the carboxy group of the C-terminal amino acid in the B-chain have a protracted profile of action.
Owner:NOVO NORDISK AS
Cyclase inhibiting parathyroid hormone antagonists or modulators and osteoporosis
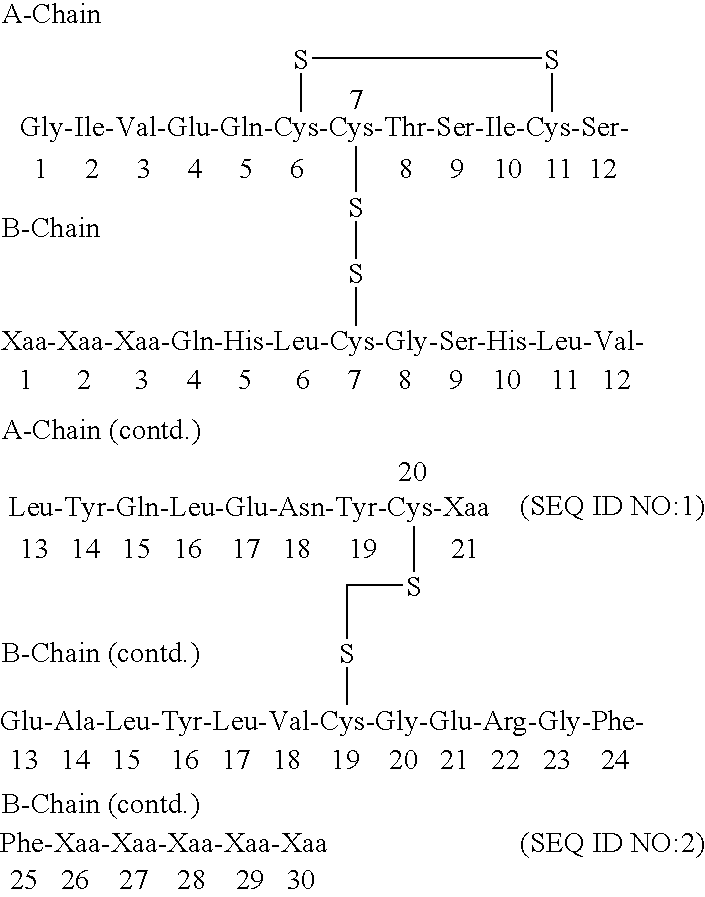
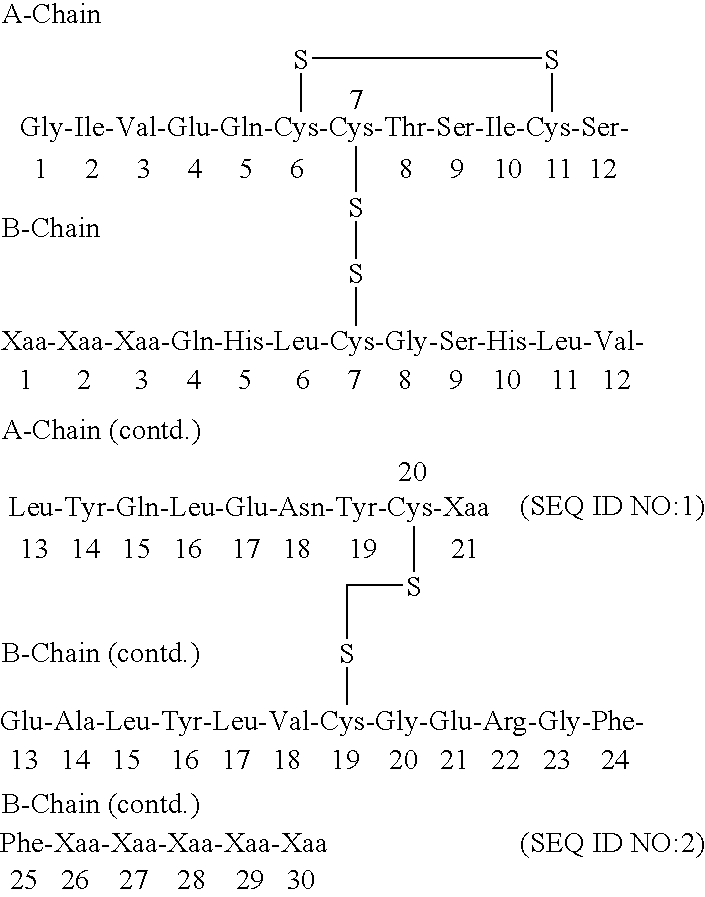
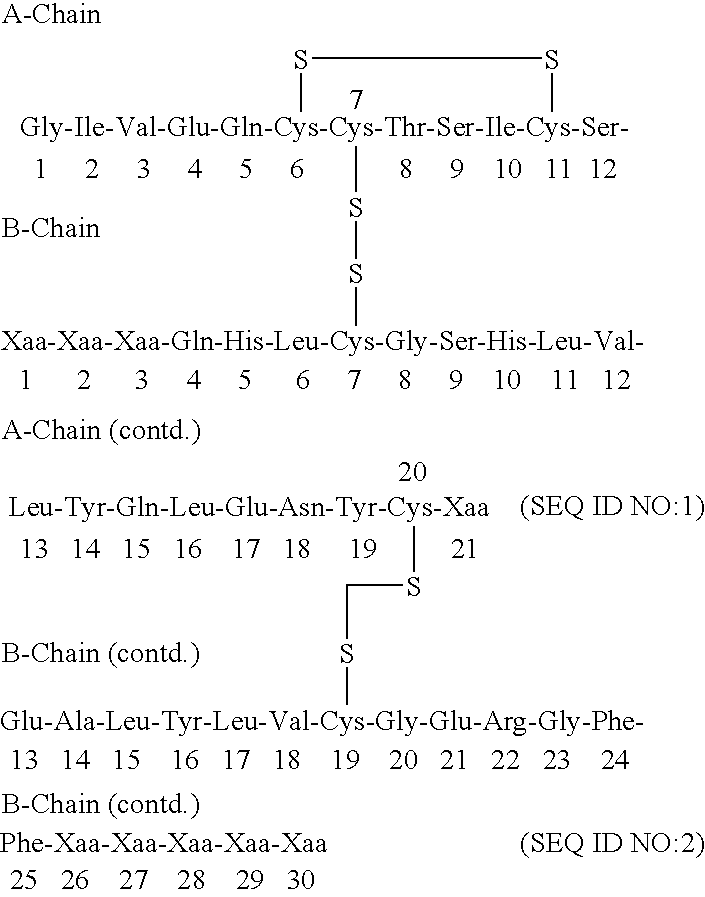
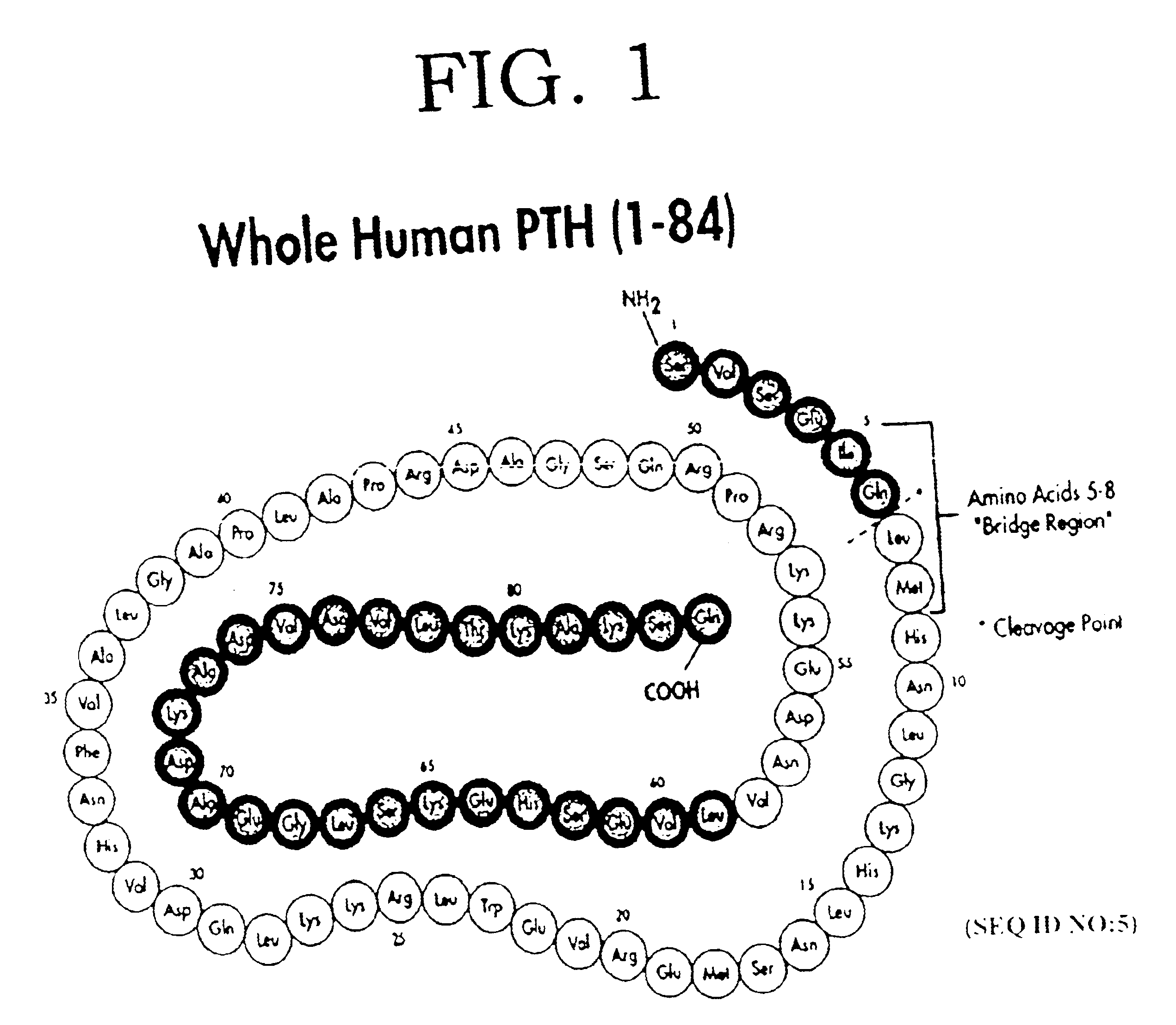
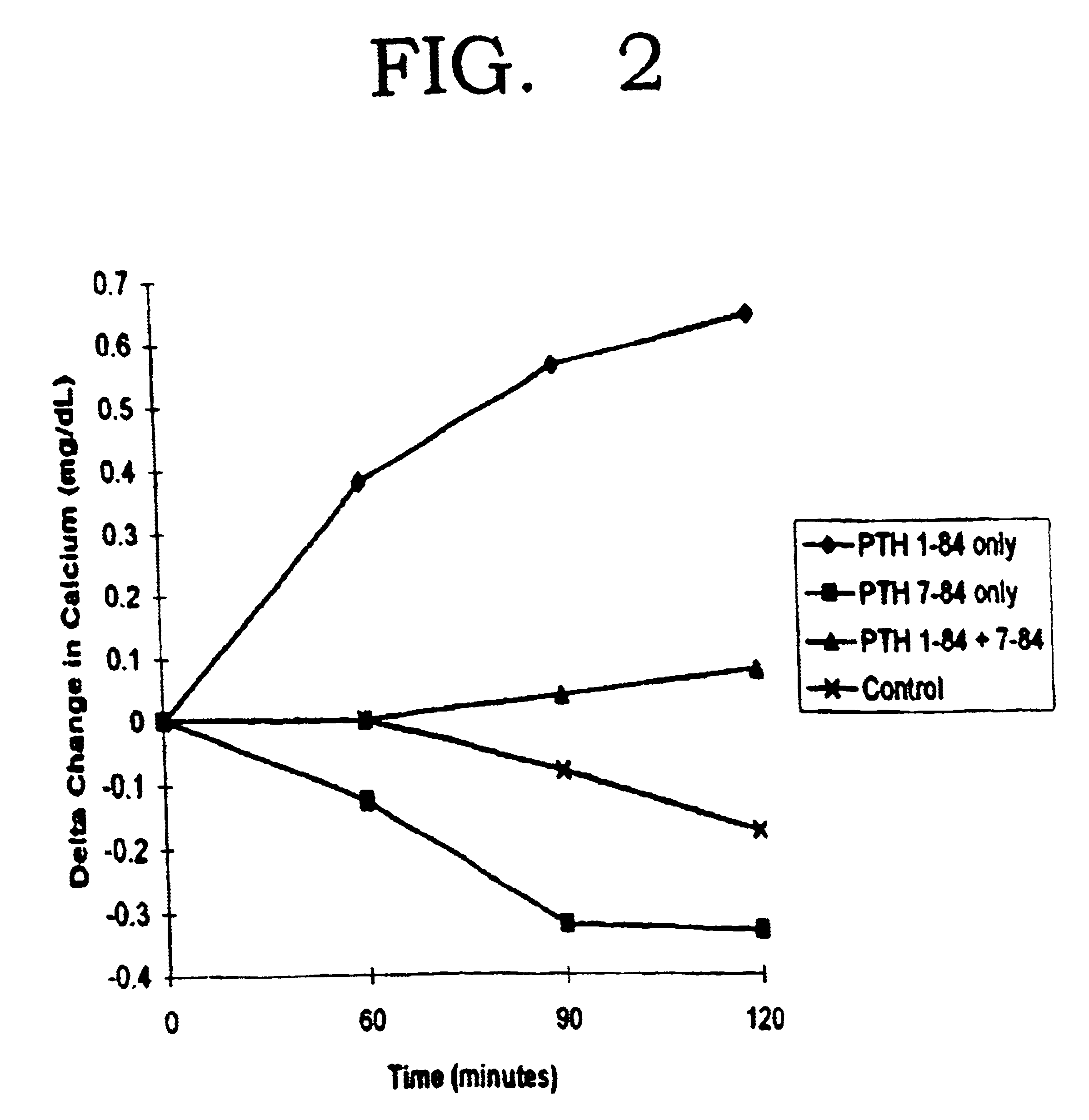
The present invention relates to a novel method for treating a patient that has osteoporosis and the patient may be having administered cyclase activating parathyroid hormone (CAP) or analogues. The patient receives an administration of a cyclase inhibiting parathyroid hormone peptide (CIP) having an amino acid sequence from between (SEQ ID NO:1 [PTH2-84]) and (SEQ ID NO:3 [PTH34-84]) (i.e., a contiguous portion of PTH having an amino acid sequence set forth in SEQ ID NO:5 (PTH1-84), having the N-terminal amino acid residue starting at any position spanning from position 2 through position 34 of the PTH1-84, and the C-terminal amino acid residue ending at position 84 of the PTH1-84), (preferably (SEQ ID NO:2 [PTH3-84]) and (SEQ ID NO:8 [PTH28-84])), or a conservatively substituted variant thereof exhibiting parathyroid hormone (PTH) antagonist activity in a therapeutically effective, but non-toxic amount that reduces the occurrence of hypercalcemia or osteosarcoma in the patient resulting from the administration of CAP, and yet, through a CAP rebound effect, is effective in itself in the treatment of osteoporosis.
Owner:SCANTIBODIES LAB
Antimicrobial amino acid sequences derived from alpha-melanocyte-stimulating hormone
InactiveUS20060111300A1Reduced viabilityAccelerate the accumulation processBiocideAntimycoticsDiseaseAmino acid
Owner:ZENGEN
Antibodies comprising c-terminal light chain polypeptide extensions and conjugates and methods of use thereof
ActiveUS20170008970A1Reducing sulfhydryl groupCyclic stabilityAntibody ingredientsImmunoglobulinsAntibody conjugateAmino acid
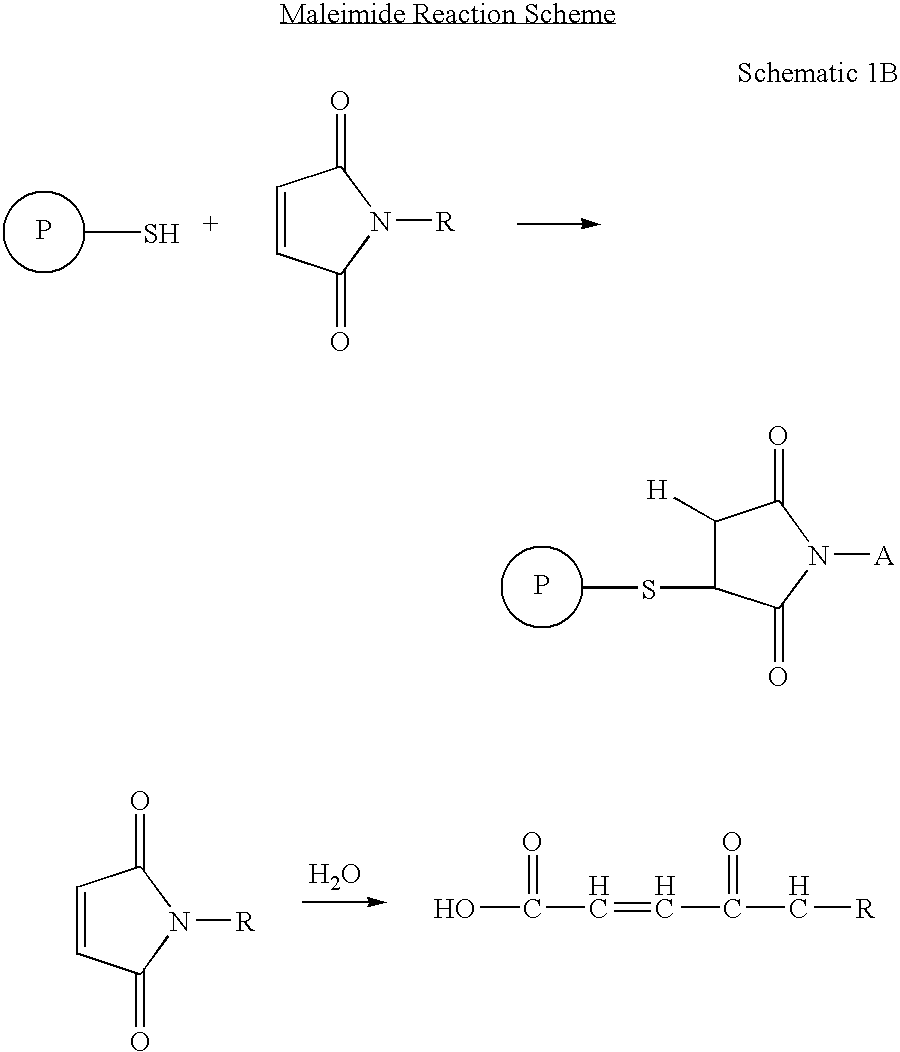
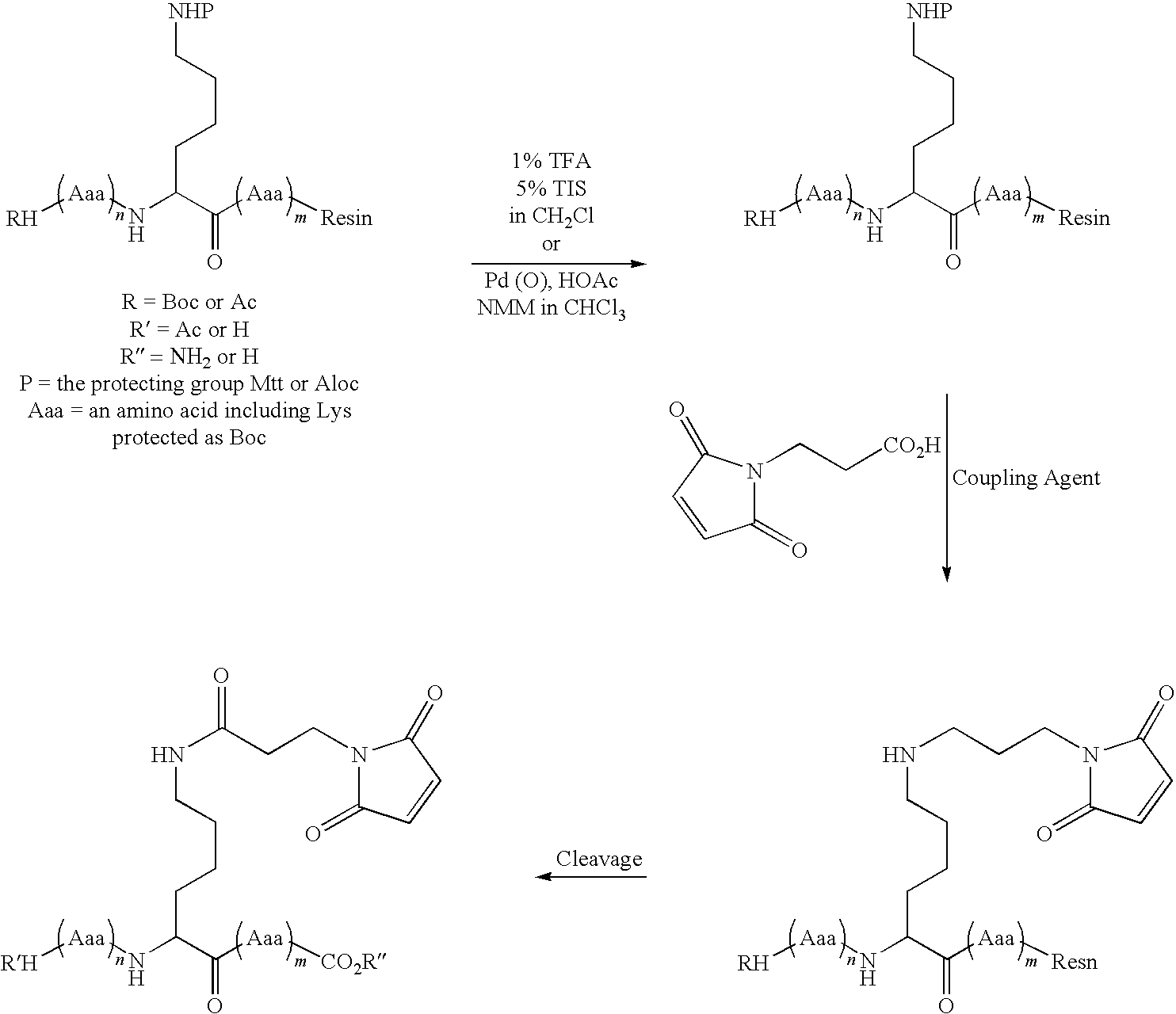
The present disclosure provides light chain polypeptides that include a C-terminal extension, as well as antibodies and antibody conjugates containing such modified light chain polypeptides, where the C-terminal extension includes one or more cysteine residues. Conjugates that include an antibody of the present disclosure conjugated to an agent via the cysteine residue of the C-terminal amino acid extension are also provided. The present disclosure further provides nucleic acids encoding an antibody light chain polypeptide that includes a C-terminal amino acid extension including a cysteine residue. Pharmaceutical compositions including the antibodies or conjugates of the present disclosure are also provided, as are methods of making and use of the modified anti-bodies and conjugates of the present disclosure.
Owner:ZYMEWORKS INC
O-phosphoserine sulfhydrylase mutants and method for production of cysteine using the same
ActiveUS9127324B2Improve enzymatic activityHigh yieldSugar derivativesBacteriaMycobacterium smegmatisO-phosphoserine sulfhydrylase
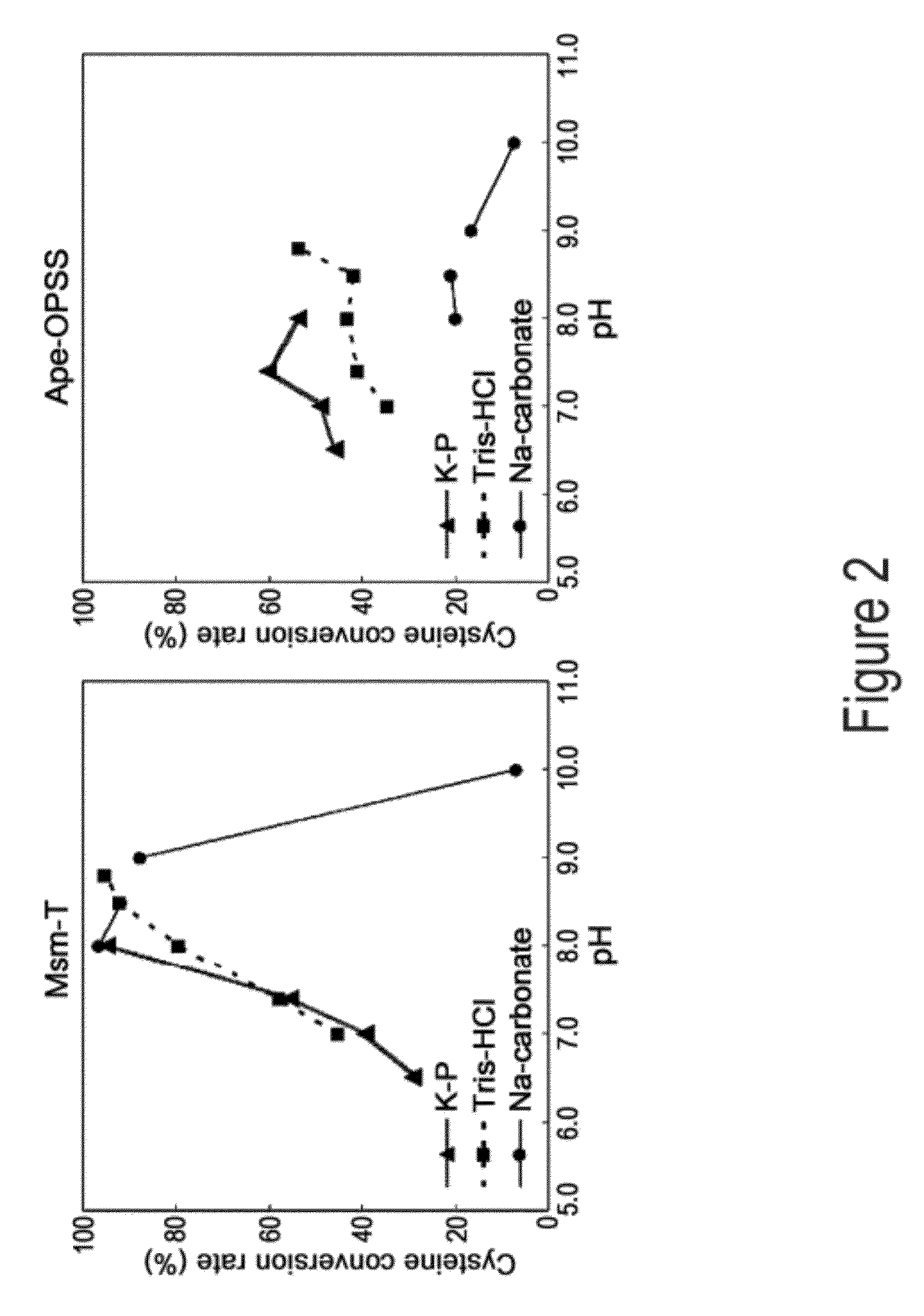
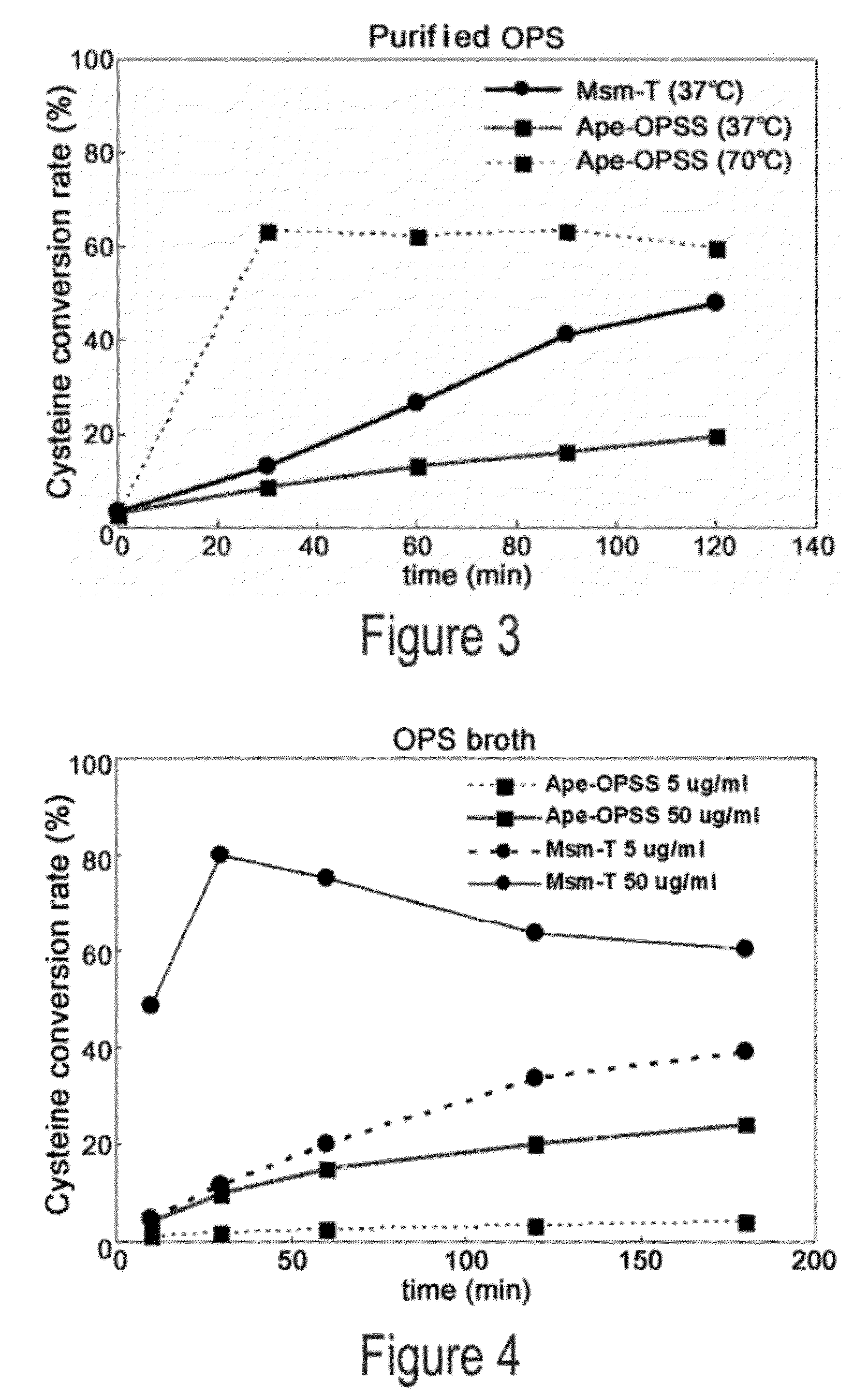
Disclosed is an O-phosphoserine sulfhydrylase (OPSS) mutant which has a Mycobacterium smegmatis-derived amino acid sequence corresponding to that of SEQ ID NO: 1 which is devoid of three to seven C-terminal amino acid residues. Also, a nucleic acid molecule encoding the OPSS mutant, an expression vector carrying the nucleic acid molecule, and a transformant transformed with the expression vector are disclosed. In addition, a method is provided for producing cysteine in which O-phospho-L-serine (OPS) is reacted with a sulfide in the presence of the OPSS mutant. The OPSS mutant has improved enzymatic activity and can be applied to the environmentally friendly production of L-cysteine through a simple enzymatic conversion reaction.
Owner:CJ CHEILJEDANG CORP
Method for Producing Peptide Thioester
InactiveUS20110184148A1Low degree of racemizationImprove stabilityPeptide/protein ingredientsPeptide preparation methodsThioester synthesisCombinatorial chemistry
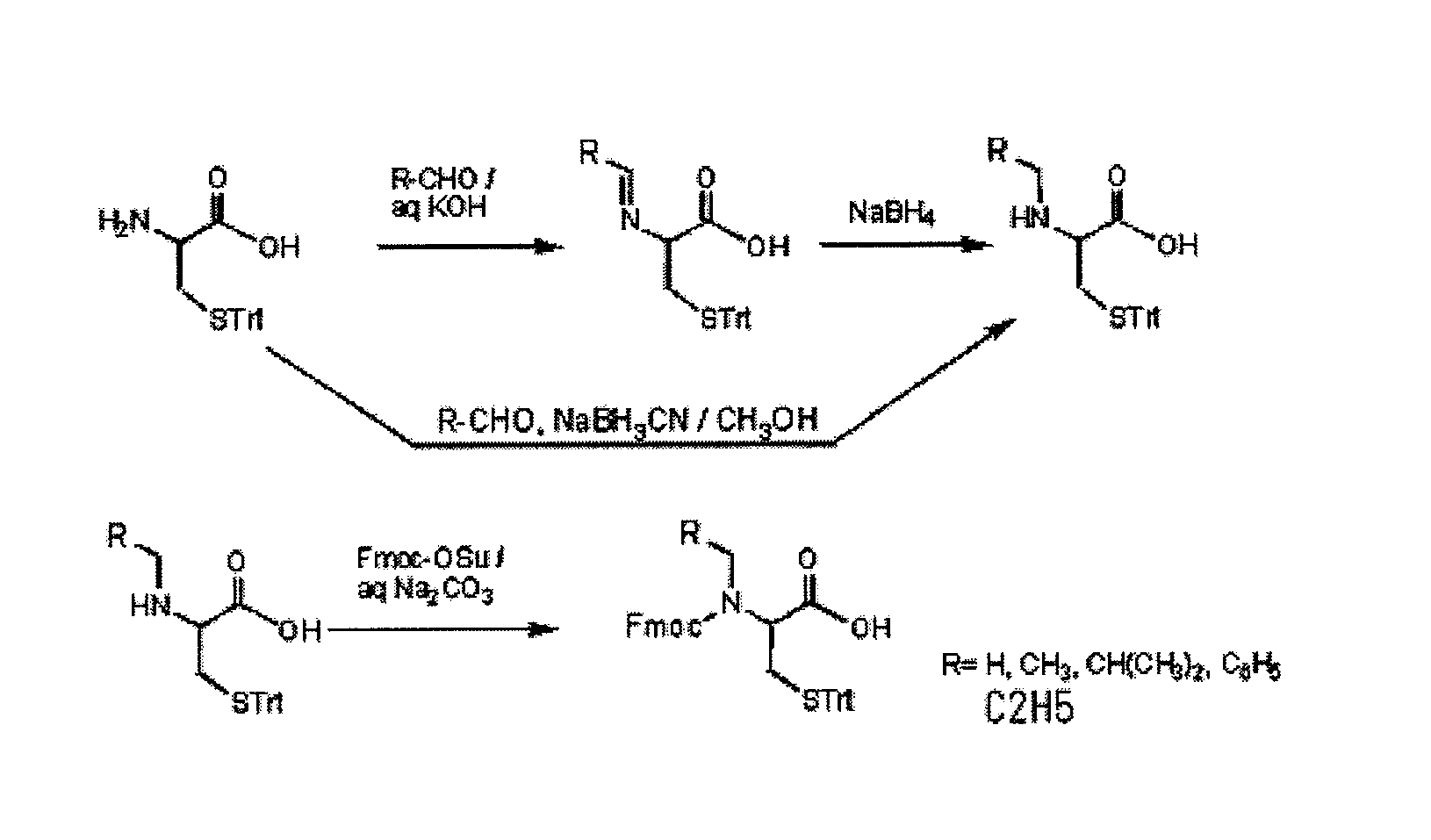
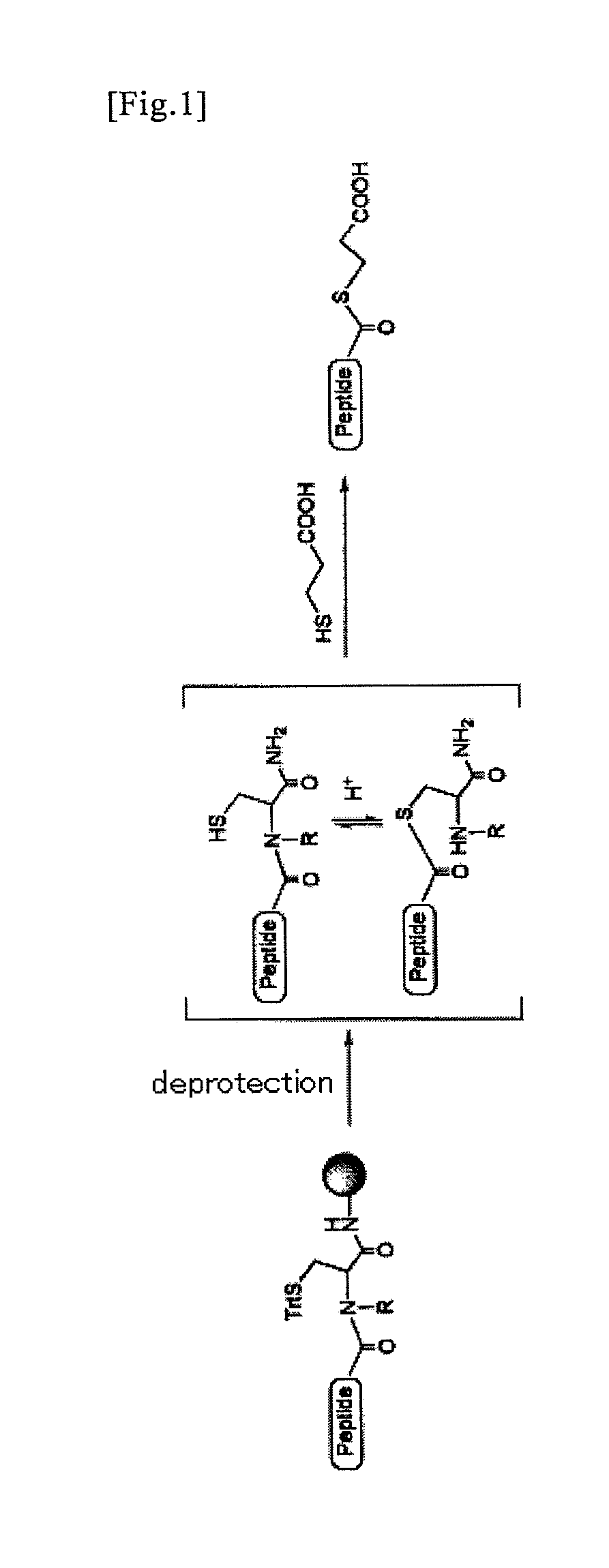
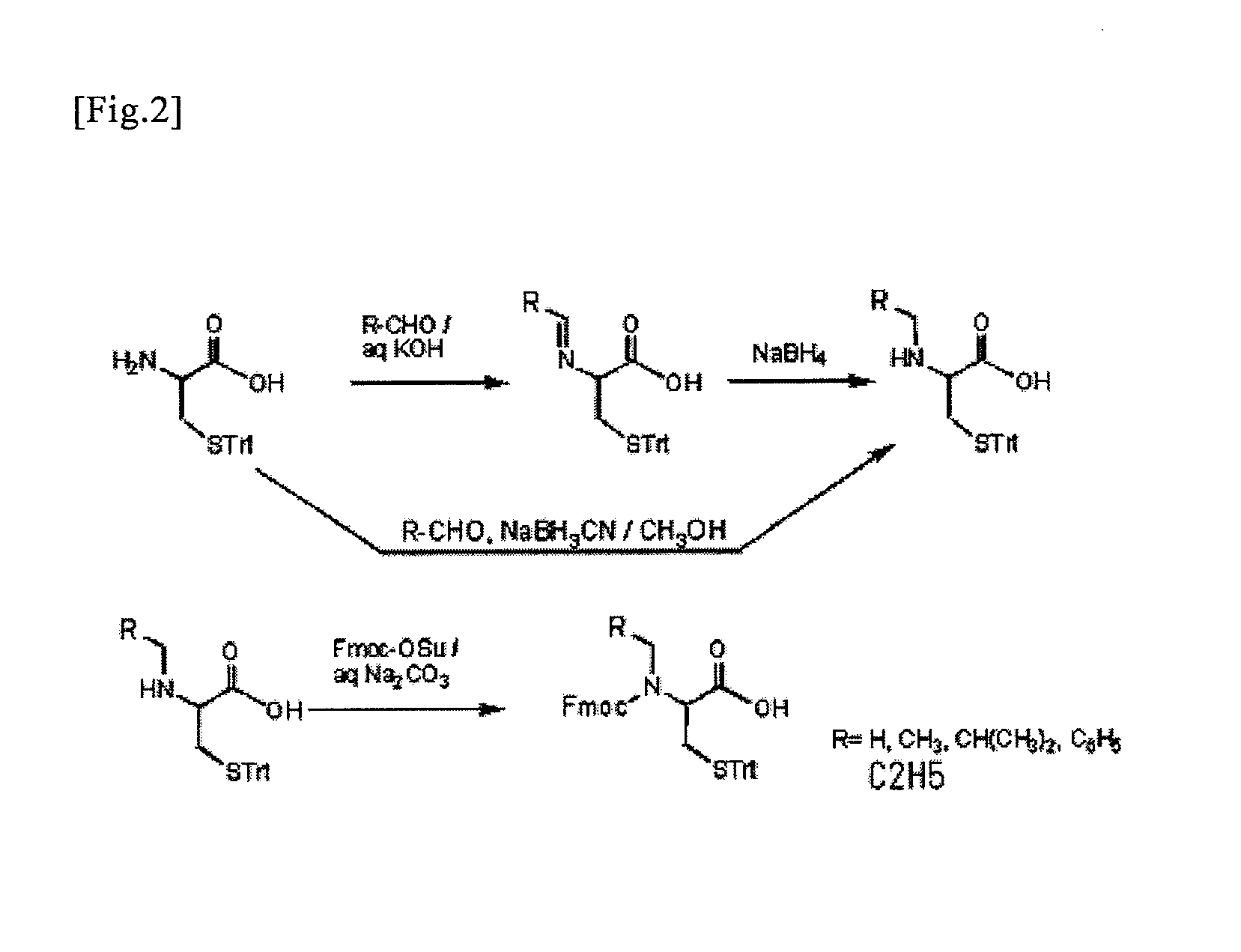
An object of the present invention is to provide a method for synthesizing a peptide thioester by using a compound that can be easily obtained within a relatively short time under conditions in which a side reaction is unlikely to occur. In the present invention, a thioester bond is formed by elongating a peptide chain using N-alkyl cysteine as the C-terminal amino acid according to the Fmoc method, carrying out deprotection, and then causing the peptide bond to undergo N—S transfer to the thiol group of N-alkyl cysteine under weak acidic conditions.
Owner:TOKAI UNIV
Rapidly degrading GFP-fusion proteins and methods of use
InactiveUS6956112B2Faster turnaroundRapid destabilizedFusion with DNA-binding domainAntibody mimetics/scaffoldsHalf-lifeADAMTS Proteins
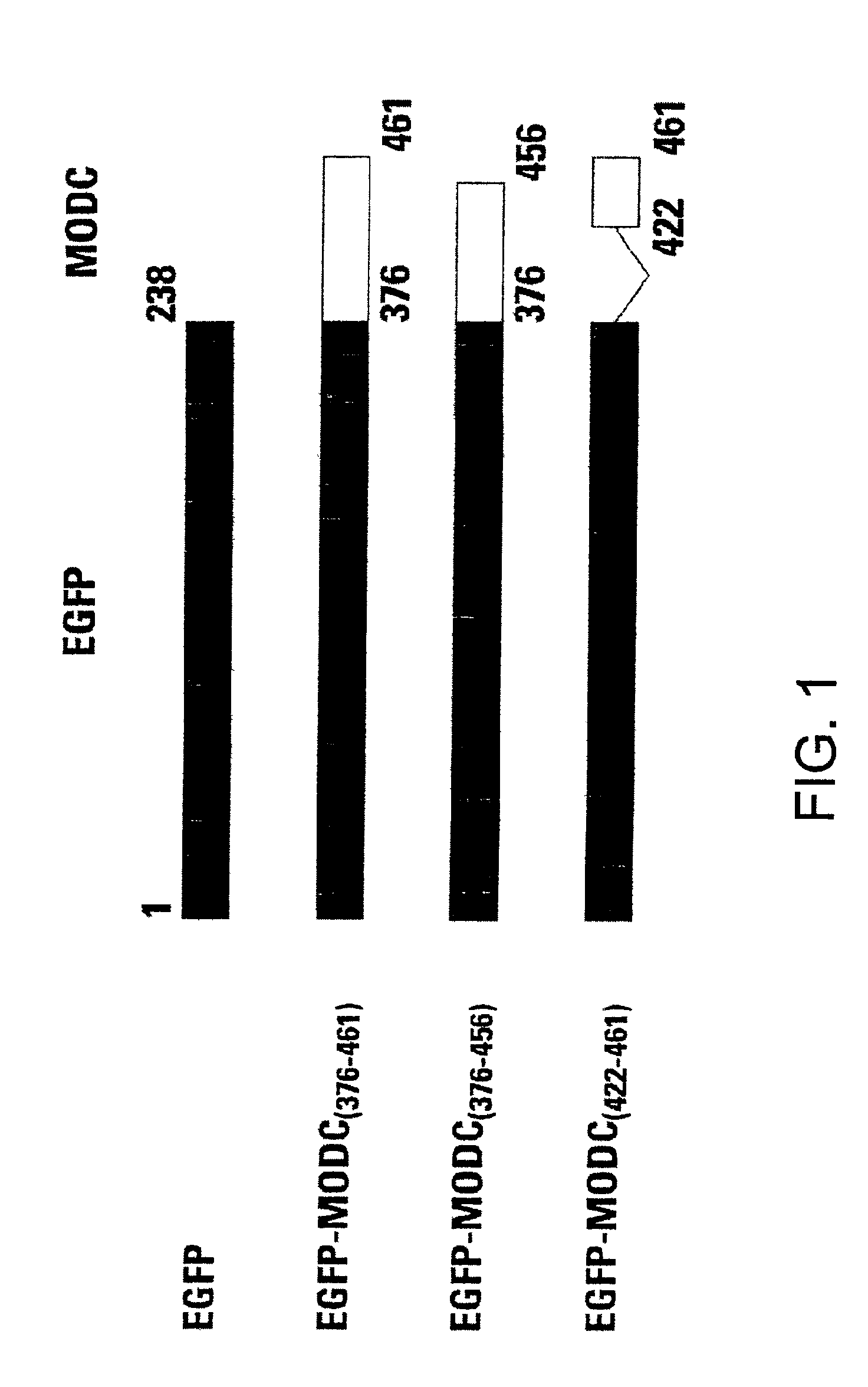
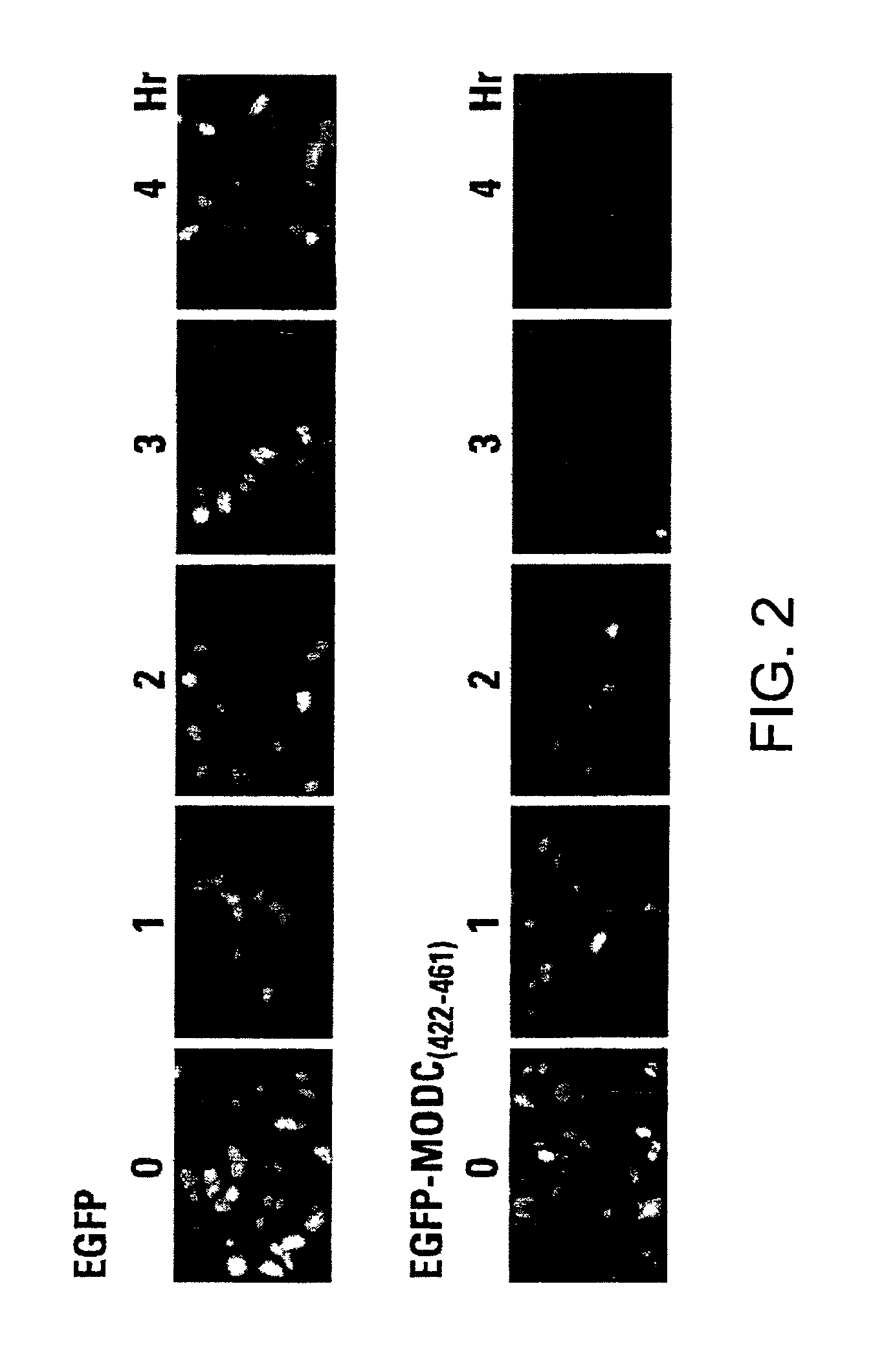
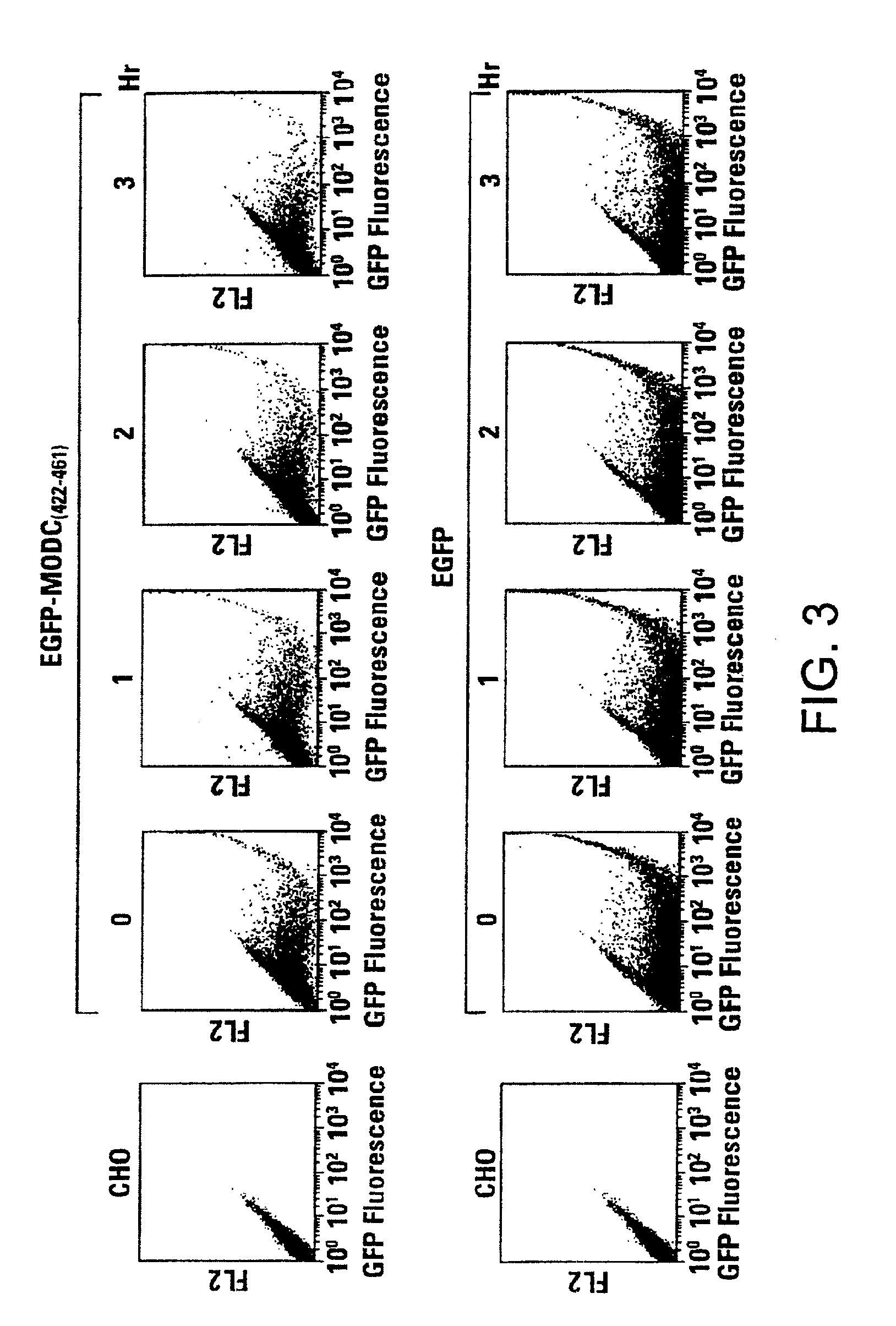
Green fluorescent protein (GFP) is widely used as a reporter in determining gene expression and protein localization. The present invention provides fusion proteins with a half life of ten hours or less with several embodiments having half lives of 4 hours or less. Such proteins may be constructed by fusing C-terminal amino acids of the degradation domain of mouse ornithine decarboxylase (MODC), which contains a PEST sequence, to the C-terminal end of an enhanced variant of GFP (EGFP). Fluorescence intensity of the fusion protein in transfected cells is similar to that of EGFP, but the fusion protein, unlike EGFP, is unstable in the presence of cycloheximide. Specific mutations in the MODC region have resulted in mutants with varying half lives, useful for a variety of purposes.
Owner:TAKARA BIO USA INC
CELL CULTURE MEDIUM AND PROCESS FOR CONTROLLING a-AMIDATION AND/OR C-TERMINAL AMINO ACID CLEAVAGE OF POLYPEPTIDES
ActiveUS20160201028A1Reduce generationRaise the ratioHydrolasesUnicellular algaeTrace elementCell culture media
The present invention is related to a cell culture medium for reducing the C-terminal heterogeneity of a polypeptide expressed in cell-culture, wherein the medium comprises at least one essential trace element in an effective amount, and to a cell culture process for reducing C-terminal heterogeneity of a protein, in which process an essential trace element is used.
Owner:LEK PHARMA D D
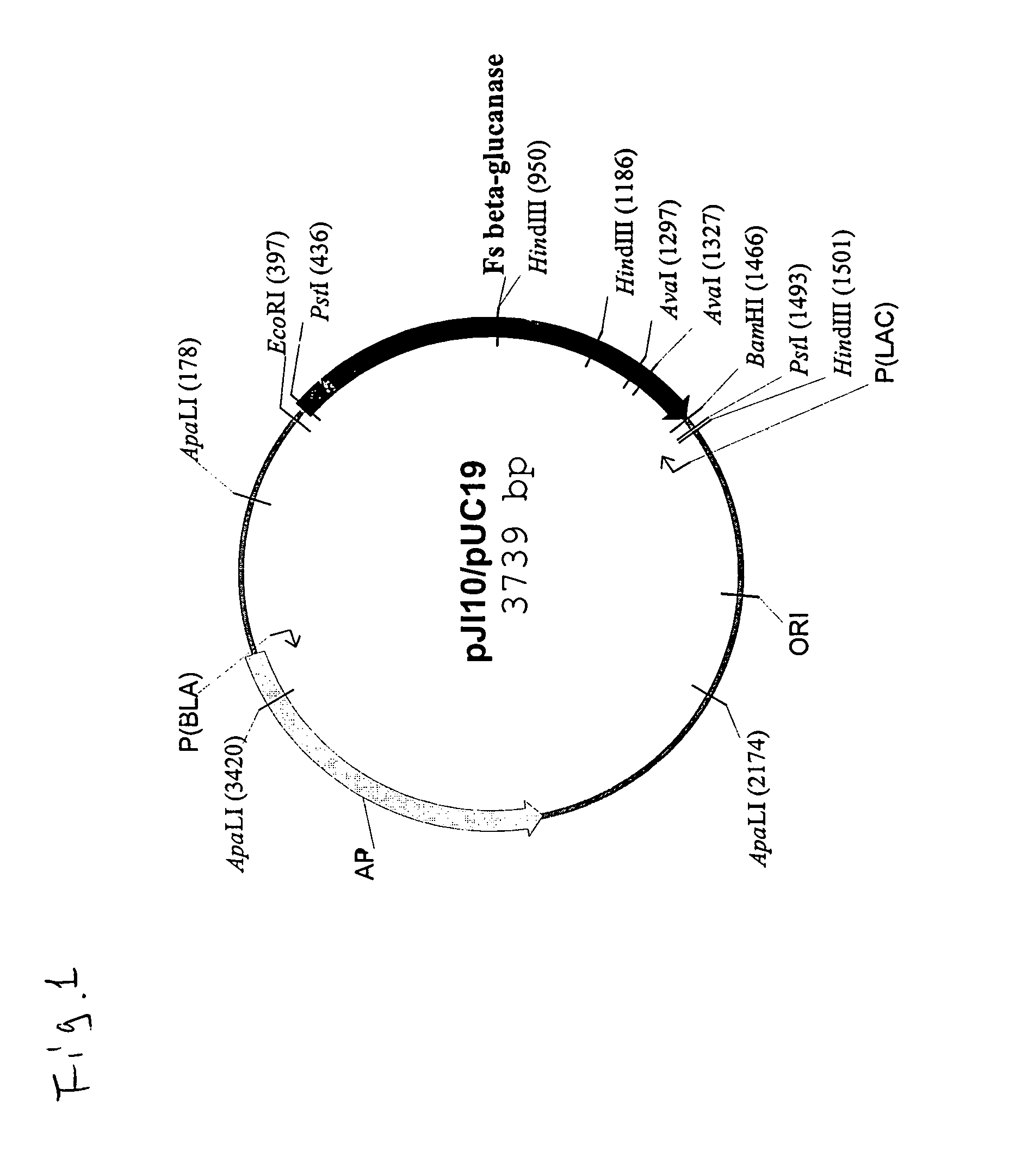
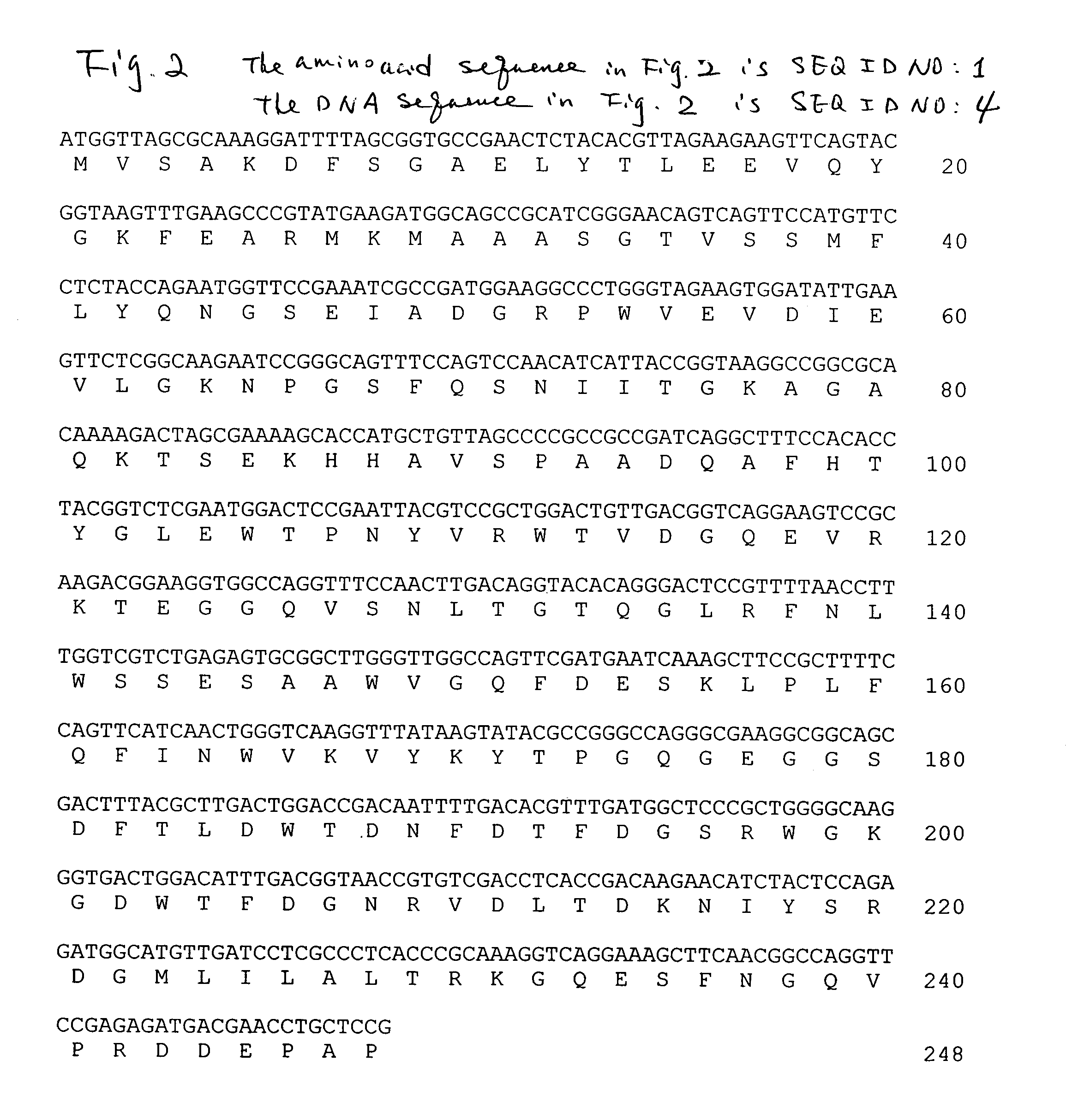
Truncated form of fibrobacter succinogenes 1,3-1, 4-β-d-glucanase with improved enzymatic activity and thermo-tolerance
InactiveUS7037696B1Glucanase enzyme can be greatly enhancedImprove thermal stabilityBacteriaSugar derivativesProtein insertionSuccinic acid
A truncated glucanase with an improved thermal stability and a higher specific enzymatic activity than the wild-type enzyme. The truncated glucanase is obtained by removing a number of amino acid residues from the C-terminal of the wild-type 1,3-1,4-β-D-glucanase of Fibrobacter succinogenes. The removal of the C-terminal amino acid residues can be conducted at the genetic level by modifying the gene encoding for the wild type enzyme using, for example, a PCR-based method. Or, it can also be conducted at the protein level by first producing the wild-type enzyme protein and then subjecting the wild-type protein to certain protease action to remove a portion of its C-terminal.
Owner:ACAD SINIC
Glucose kinase derivant for inhibiting platelet aggregation and thrombase activity, and its preparing method
ActiveCN1850976ABlock thrombin activityInhibit aggregationPeptide/protein ingredientsHydrolasesAntigenFusion Protein Expression
The invention discloses the grape dashing enzyme ramification dealing with the blood platelet assembling and the thrombin active ability and the producing process belonging to the domain of the gene engineering and the producing art of the biology dissolving bolt medicament. The nucleotide acid serial of the grape dashing enzyme ramification restraining the blood platelet assembling and the thrombin active ability is disclosed. The producing process fuses the RGD series, the C end series of the hirudin and the constructed low antigen and high active ability SAK breaking body gene using the gene recombining technology outer the body, at the same time, the Xa gene incising location point and the flexible connecting arm and it's C end amino acid is decorated, the another amino acid is appended. The fusing albumen expressing carrier is constructed to gain the efficient expressed recombining SAK ramification using the double enzyme chipping and the PCR appraising alkalescence of the constructed breaking body. The ramification has the function of restraining the blood platelet assembling and interdicting the thrombin active ability and establishes the base of developing the new generation dissolving bolt medication. So the applied range of the new SAK ramification is expanded largely.
Owner:INST OF BASIC MEDICAL SCI ACAD OF MILITARY MEDICAL SCI OF PLA
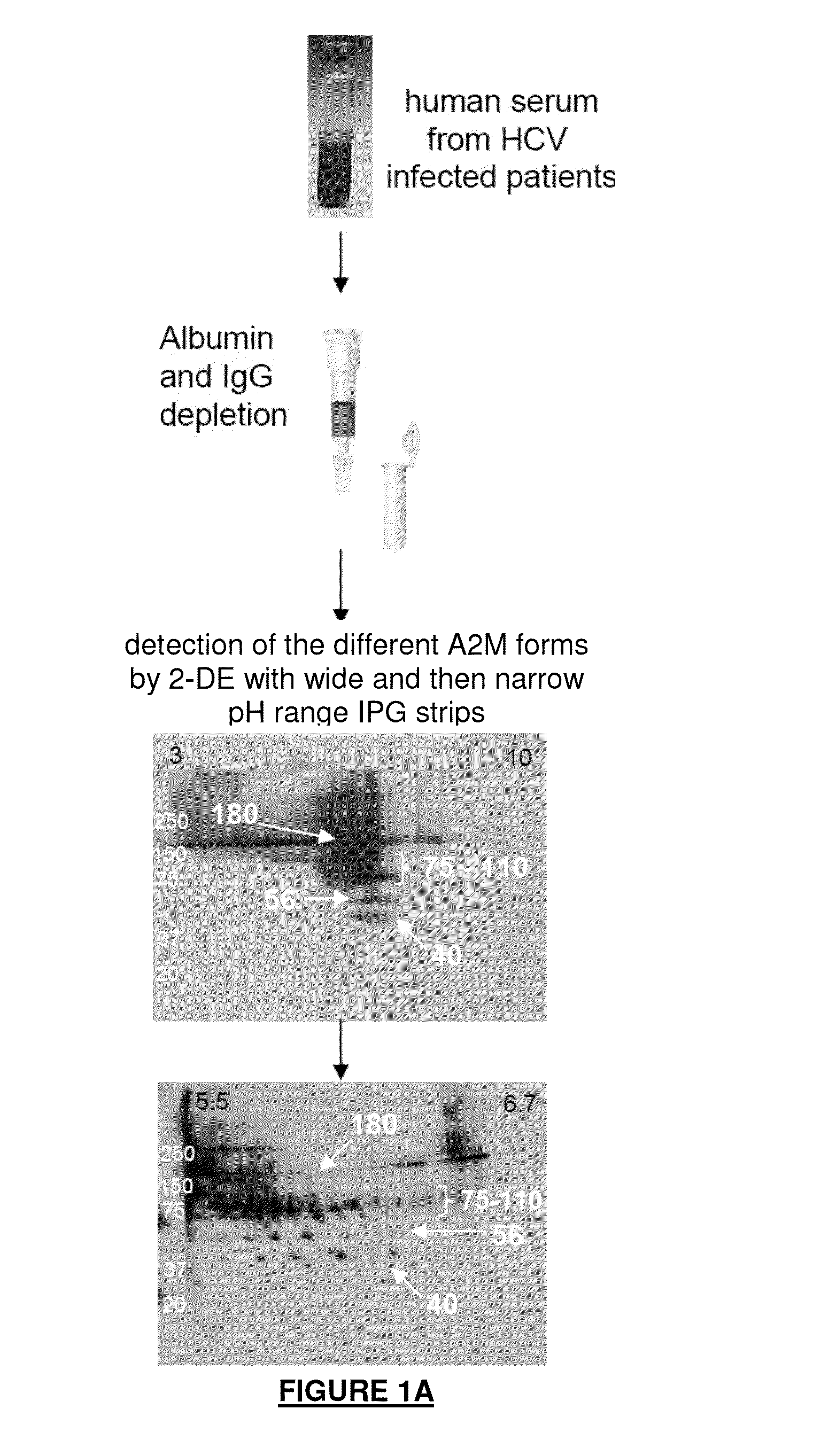
A2m fragments and applications thereof
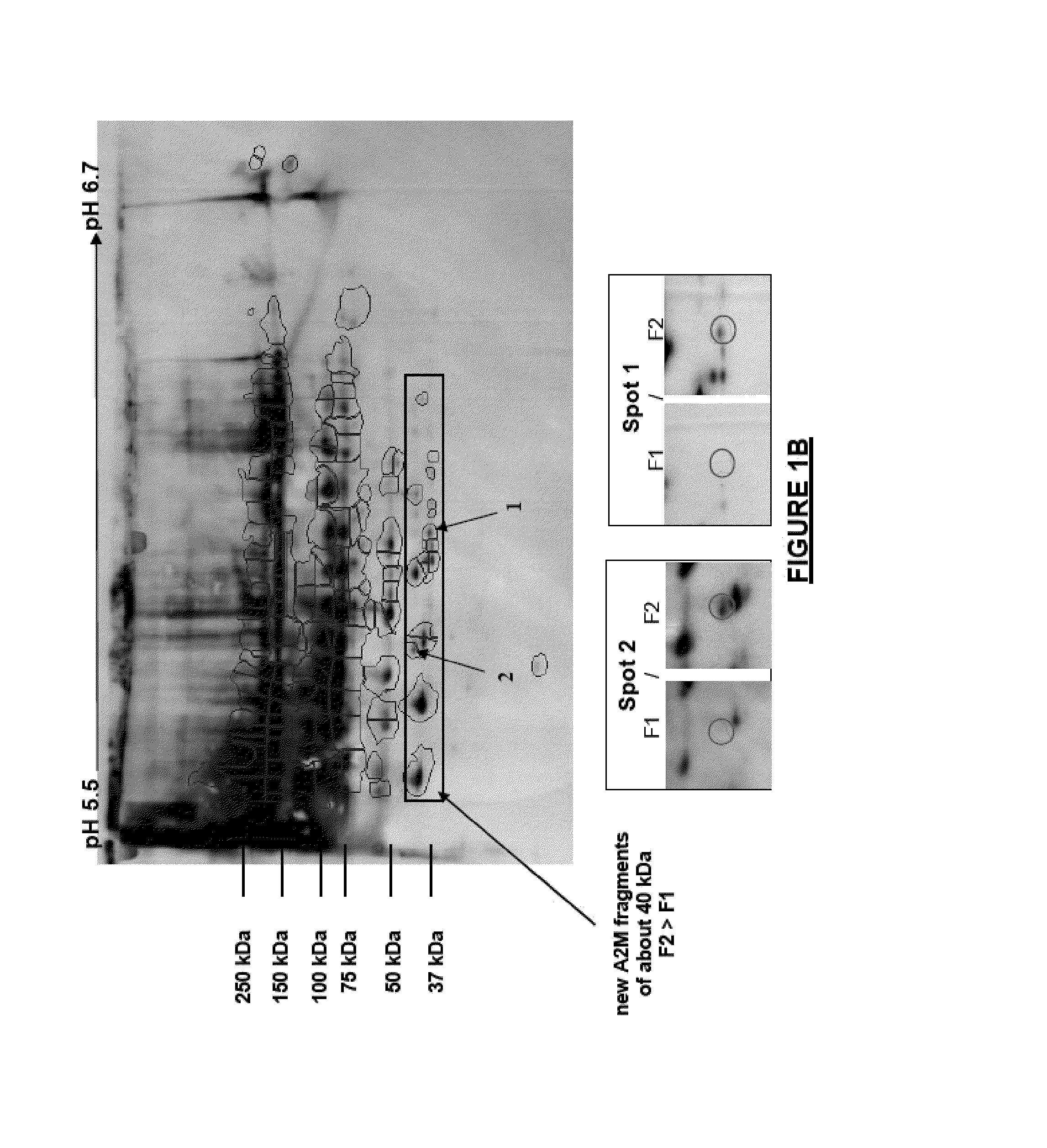
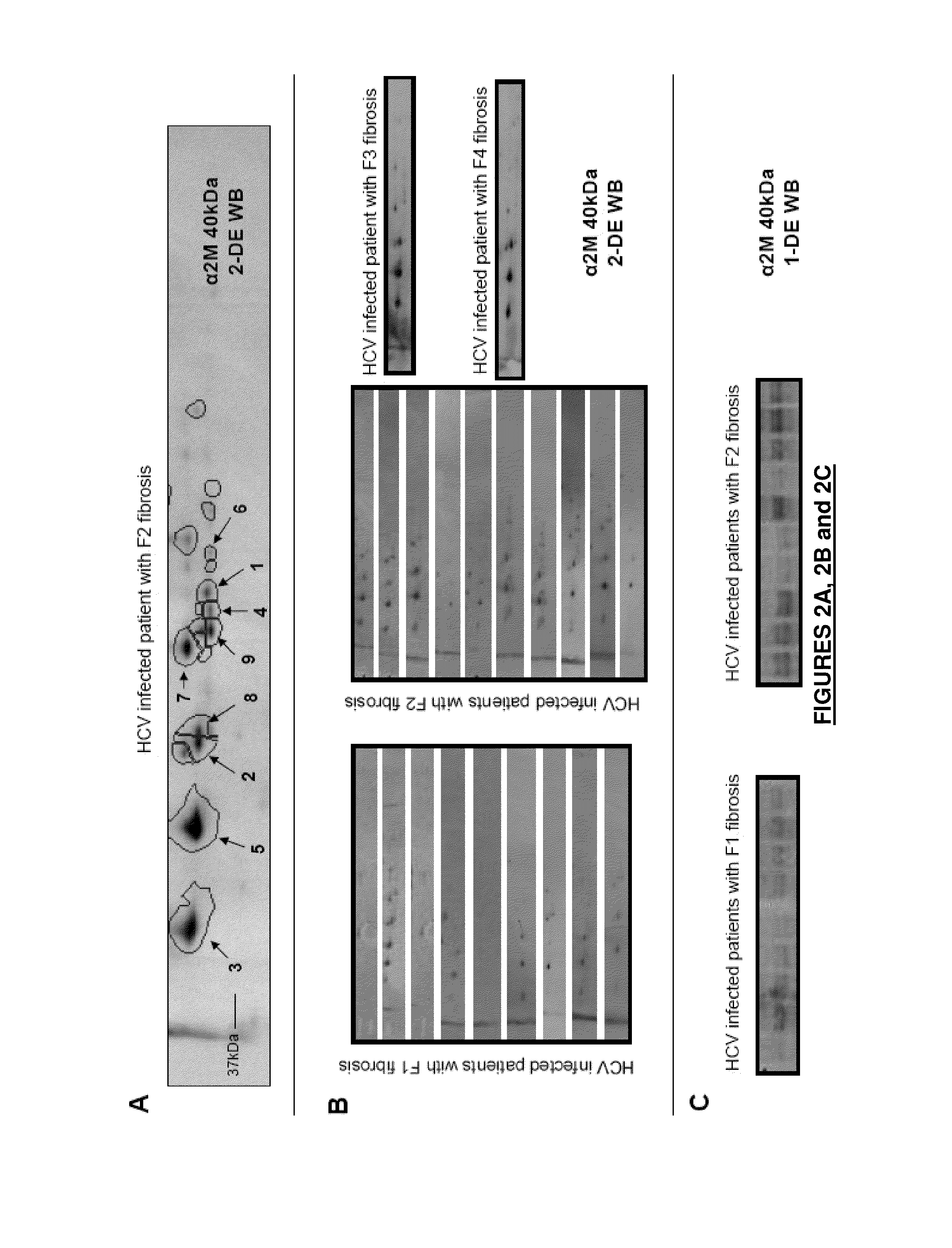
The application relates to a polypeptide, the amino acid sequence of which is the sequence of a sub-fragment of the C-terminal thioester-cleaved fragment of human alpha-2-macroglobulin (A2M), wherein the molecular weight of said polypeptide is of 36 to 44 kDa, and wherein the first N-terminal amino acid of said polypeptide is an amino acid, which, in the full length sequence of said human A2M, is at a position 1,098 or 1,085 or 1,084 or 1,083, and the last C-terminal amino acid of said polypeptide is an amino acid, which, in the full length sequence of said human A2M, is one of the last twenty C-terminal amino acids. This polypeptide is differently abundant depending on the stage of liver fibrosis. The application also relates to means deriving therefrom and to the application thereof, notably in the field of hepatitis.
Owner:BIO RAD INNOVATIONS SAS +1
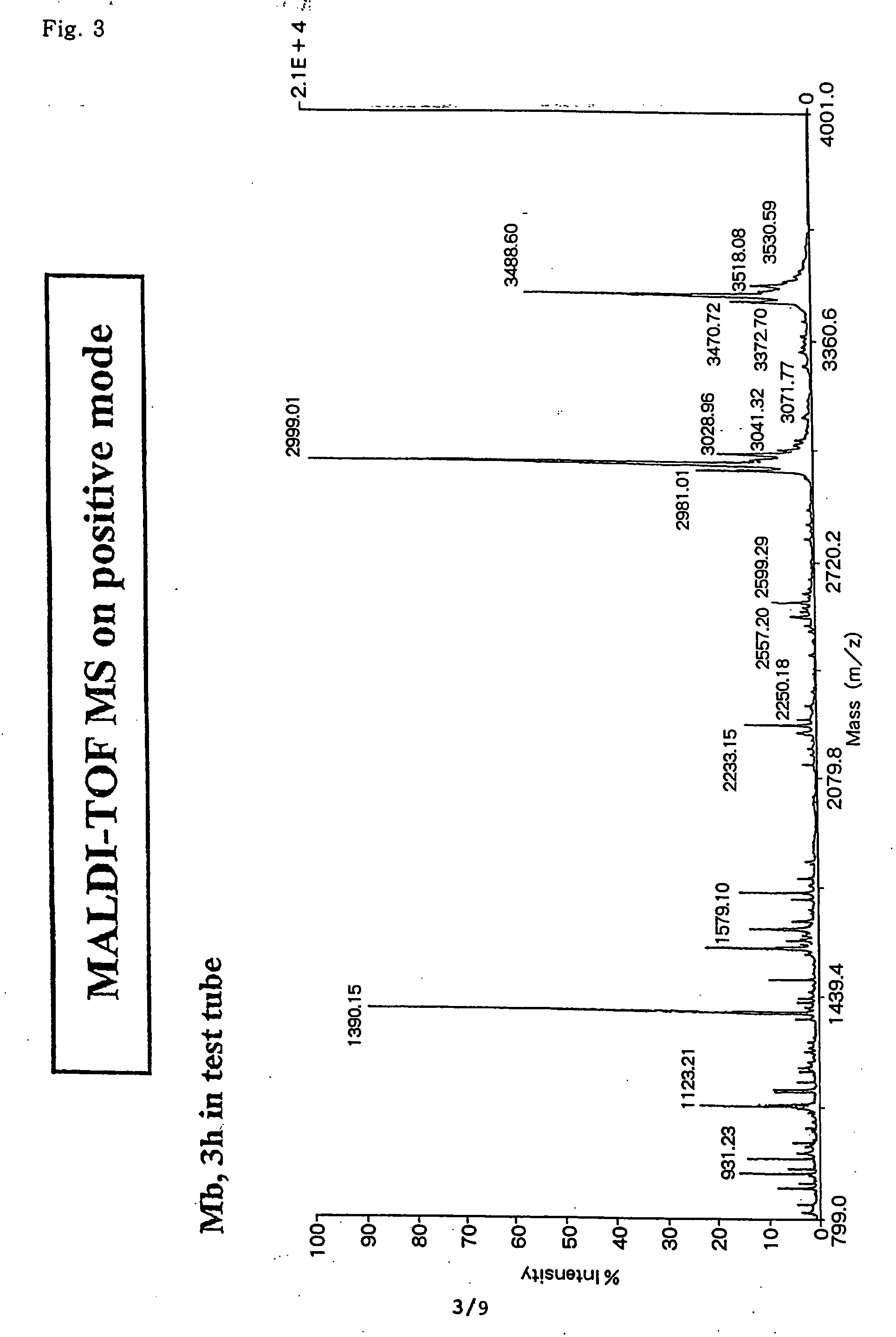
Method for analyzing c-terminal amino acid sequence of peptide using mass spectrometry
InactiveUS20060134720A1High strengthEasy to identifyPeptide/protein ingredientsMicrobiological testing/measurementChemical treatmentArginine
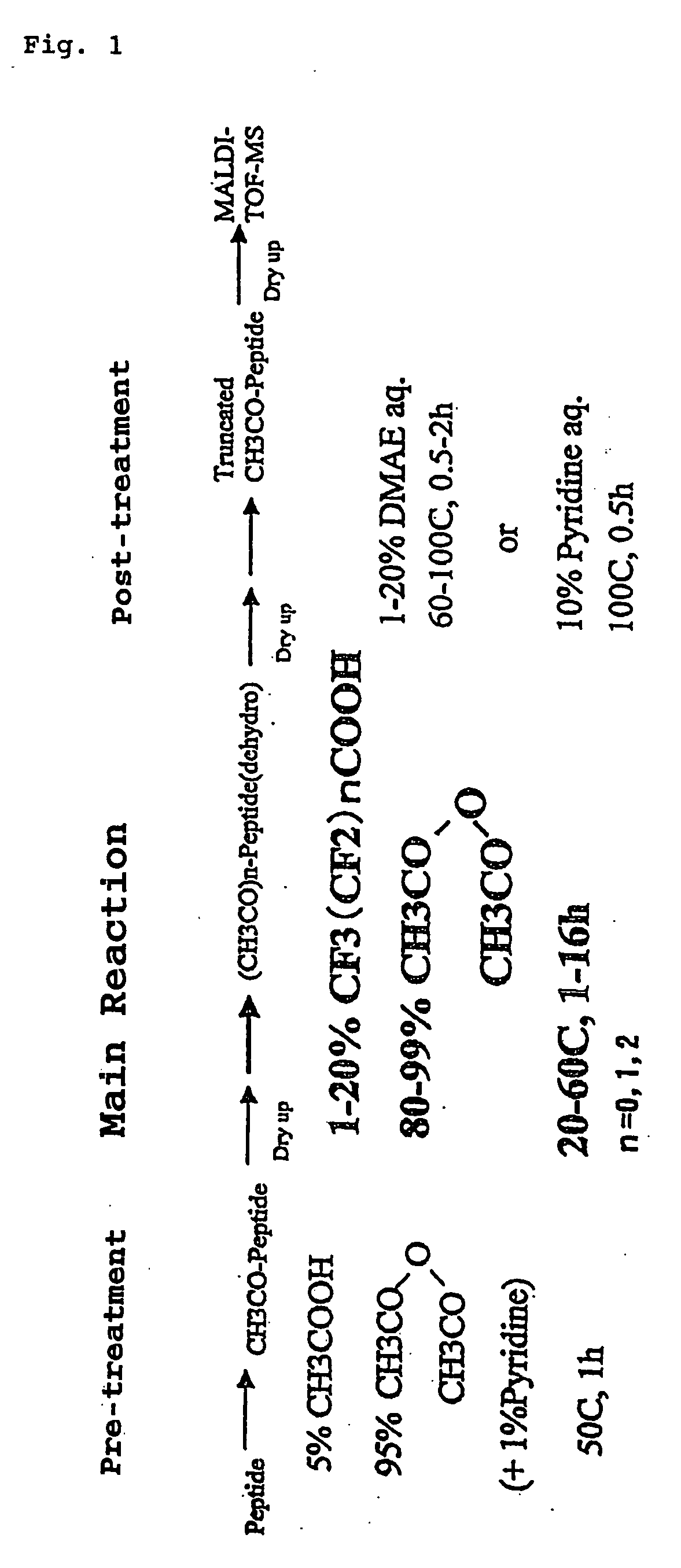
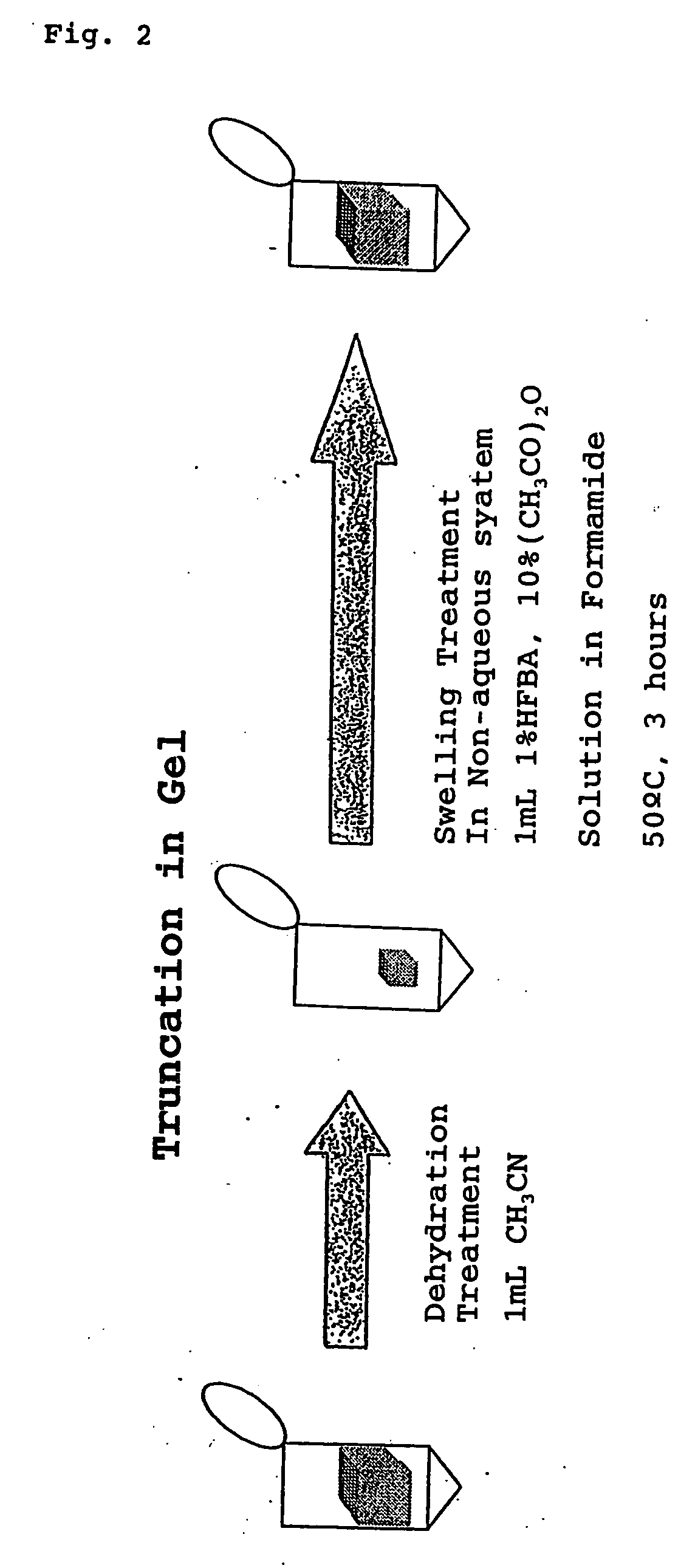
The present invention provides a method for analyzing the C-terminal amino acid sequence of a peptide by using a reaction for successively releasing the C-terminal amino acids of the peptide, which method can suppress, when successively releasing the C-terminal amino acids of a peptide of long amino acid length, such a undesirable side reaction as cleavage of peptide bond in the intermediate position of the peptide and can carry out the chemical treatment thereof under widely applicable conditions; In the method, a dry sample of a peptide with long amino acid length is beforehand subjected to an N-acylation treatment; by using a reaction reagent where an alkanoic acid anhydride is combined with a small amount of a perfluoroalkanoic acid, successive release of C-terminal amino acids is conducted under mild conditions; a hydrolysis treatment is applied; then, selective fragmentization at site of arginine residue is performed by digestion by trypsin; thereafter, decreases in molecular weight are measured for the C-terminal side fragments derived from a series of reaction products with use of a MALDI-TOF-MS apparatus; thereby, the C-terminal amino acid sequence of the peptide sample is identified.
Owner:NEC CORP
Expression Cassette, Recombinant Host Cell and Process for Producing a Target Protein
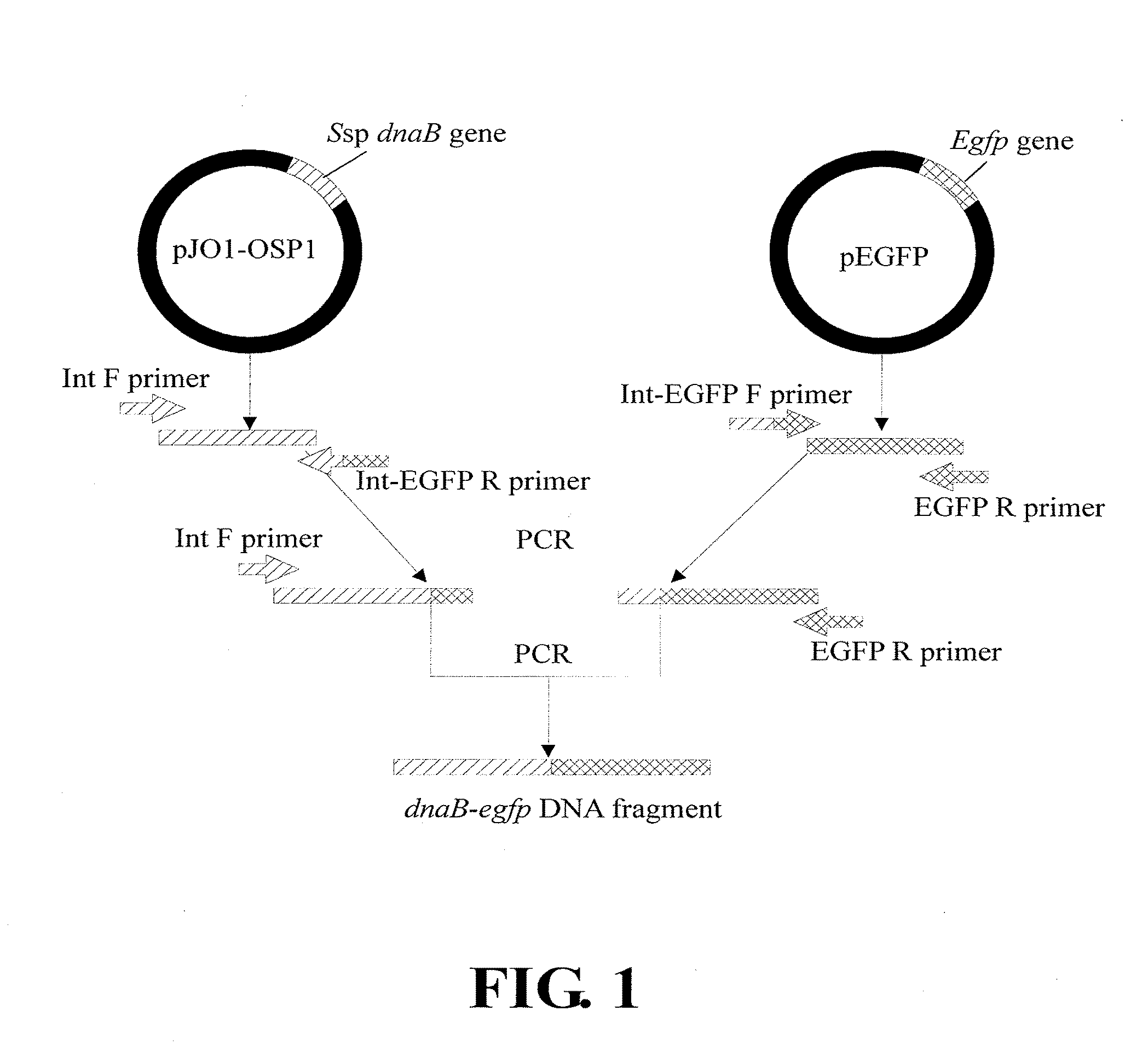
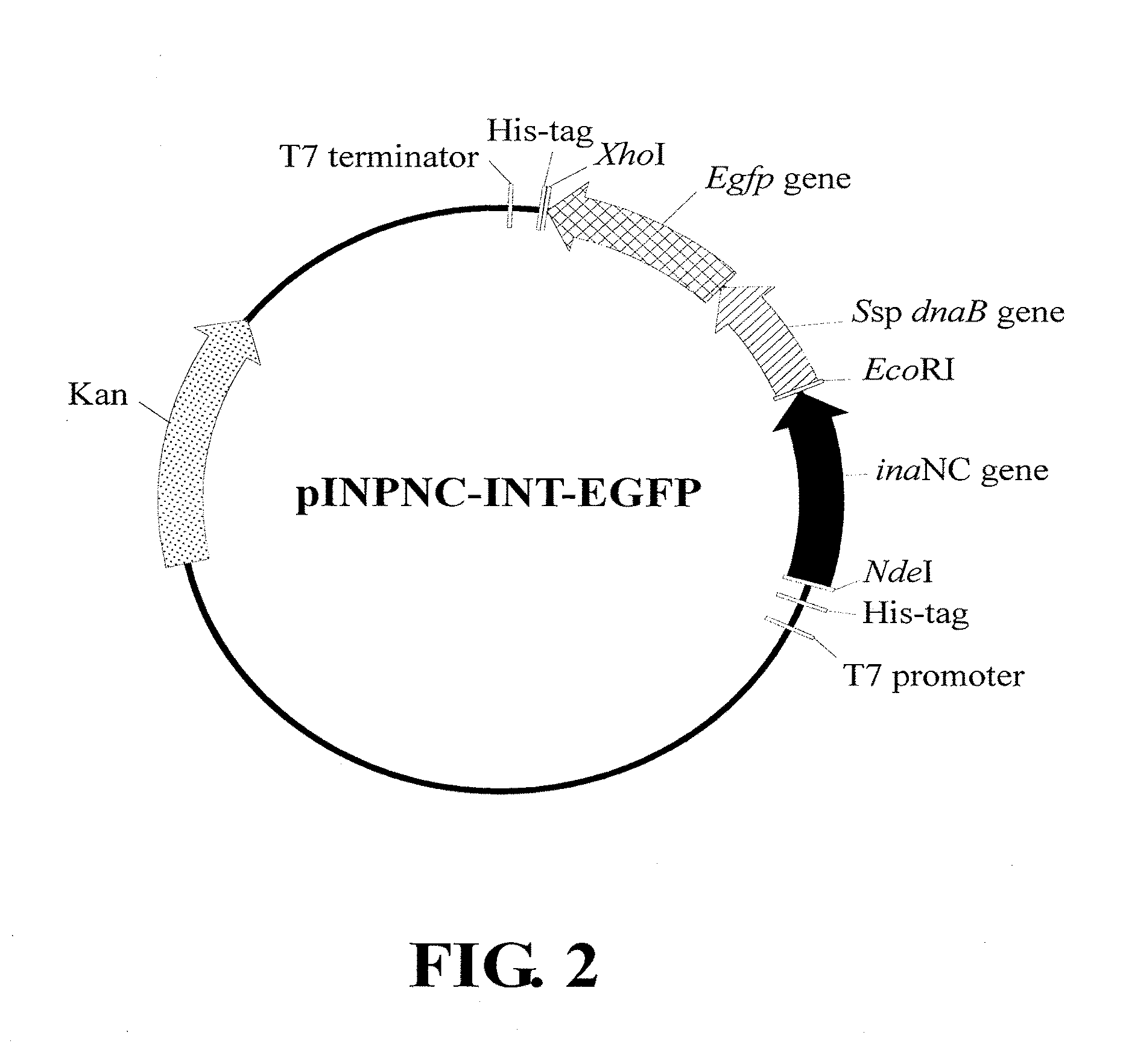
Disclosed herein is a process for producing a target protein, in which a recombinant polynucleotide is constructed to encode a fusion protein including: (i) an anchoring protein that includes a N-terminal amino acid sequence of an ice nucleation protein, so that the fusion protein, once expressed in the host cell, is directed by the anchoring protein to be anchored and exposed on the outer membrane of the host cell; (ii) the target protein; and (iii) a self-splicing protein that includes a first end fused with the anchoring protein and a second end fused with the target protein, wherein the self-splicing protein includes a N-terminal or C-terminal amino acid sequence of an intein protein at the second end thereof, such that upon an environmental stimulus, the self-splicing protein exerts a self-cleavage at the second end thereof to release the target protein from the fusion protein.
Owner:NATIONAL CHUNG HSING UNIVERSITY
Heparinase II deletion mutant coding gene and protein thereof
The invention discloses a heparinase II deletion mutant protein and a coding gene thereof. An amino acid sequence of the heparinase II deletion mutant protein provided by the invention is as shown in SEQ ID NO:2. The coding gene of the protein is also within a protective range. According to the invention, a primer is firstly designed by a PCR technology, so that heparinase II deletes a C-terminal amino acid sequence to obtain the heparinase II with smaller molecular weight and higher activity; after the heparinase II is purified, the specific activity of the heparinase II can reach 43.3IU / mg. The heparinase II deletion mutant coding gene and protein thereof disclosed by the invention can be widely applied to producing the heparinase II deletion mutant.
Owner:SHENZHEN HEPALINK PHARMA GRP CO LTD
Glycoprotein Hormone Analogs
Owner:RUTGERS THE STATE UNIV
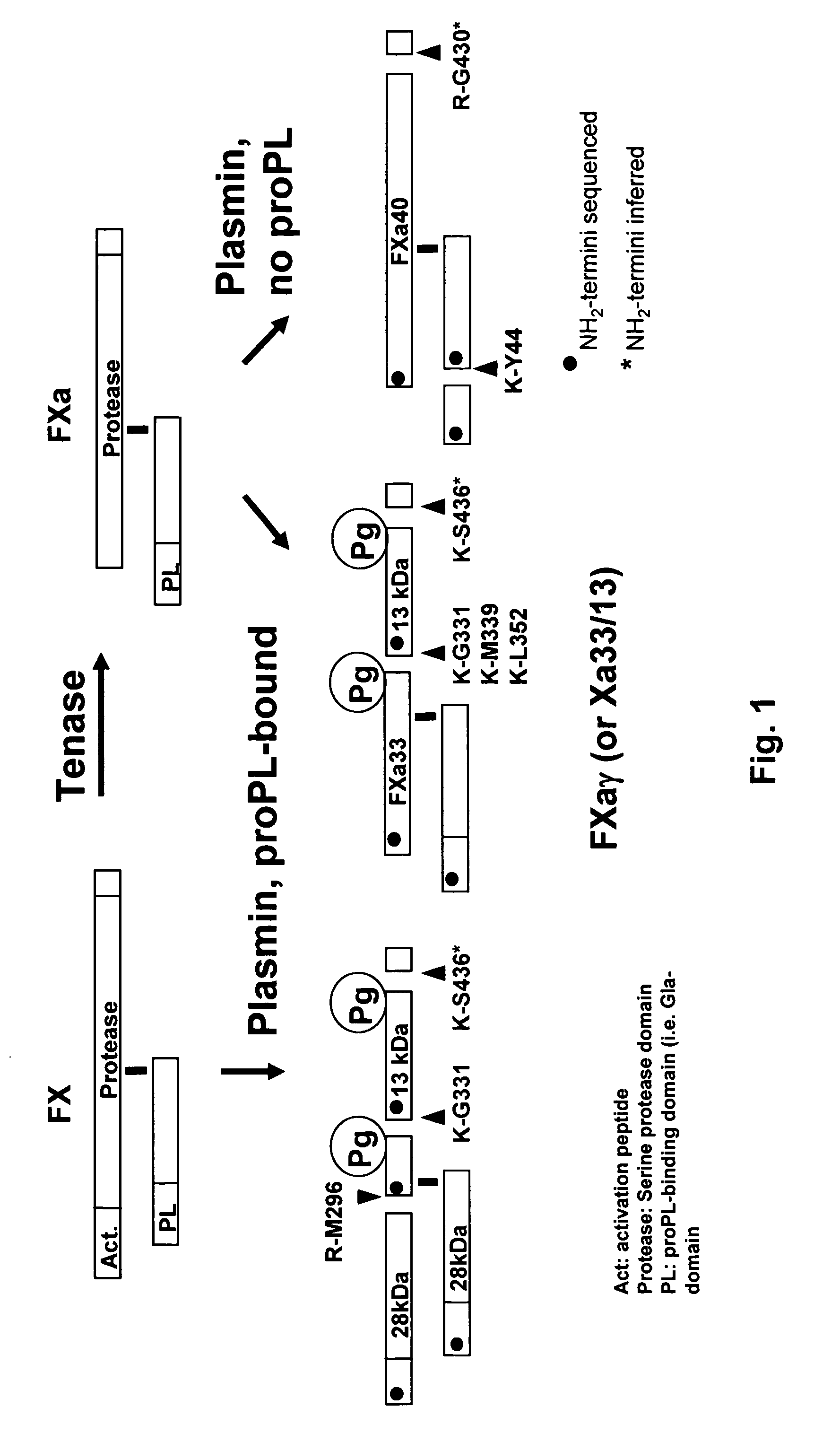
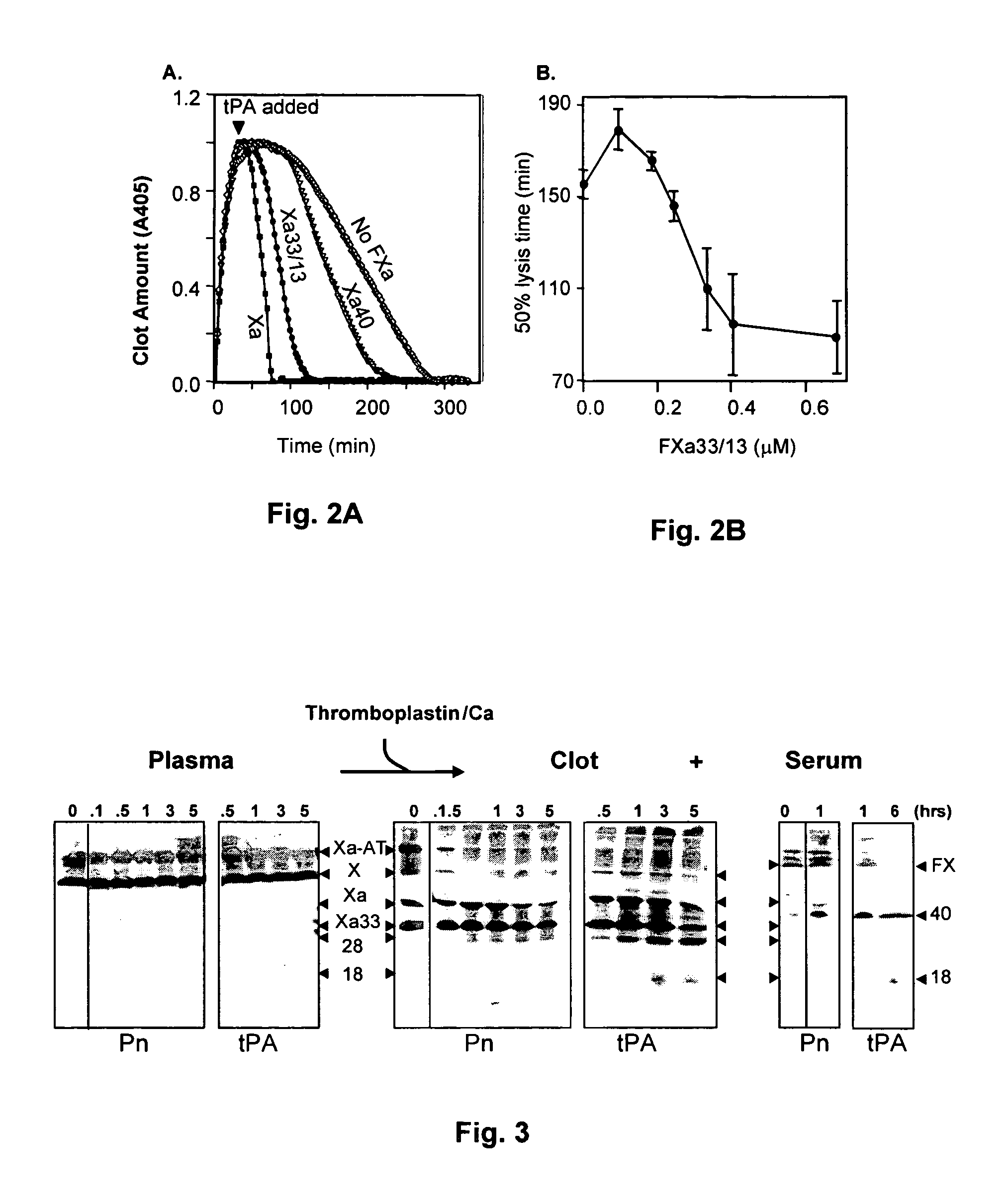
Coagulation proteins, coagulation-anticoagulation protein complexes, derivatives thereof and their uses
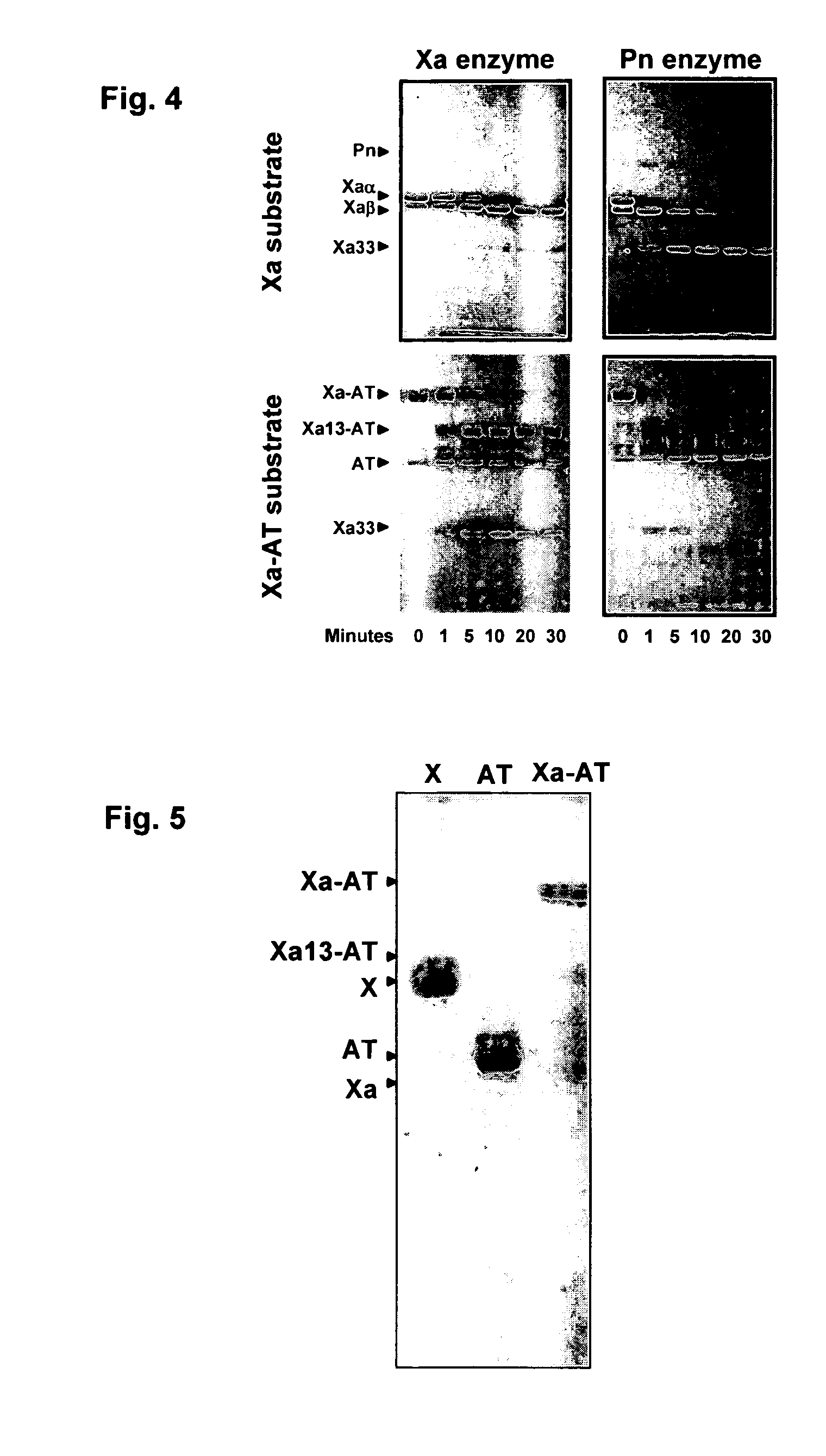
InactiveUS20070025979A1Enhance dissolving said blood clotHigh dissolution rateOrganic active ingredientsFibrinogenSide effectFactor X
The present invention relates to the use of coagulation proteins and complexes thereof with anticoagulation proteins for the lysis of blood clots or other applications affected by accelerated plasmin production. More specifically, the present invention provides a method for accelerating the dissolution of a blood clot through the administration of at least one coagulation protein, with or without being in complex with a serpin, comprising a basic C-terminal amino acid, wherein the coagulation protein may be a derivative of Factor X or Factor V or a combination thereof. Pharmaceutical compositions for the treatment and prophylaxis of blood clots are also provided, wherein, the methods and products of the present invention advantageously accelerate clot dissolution while potentially minimizing the adverse side-effects, such as hemorrhaging, seen with other clot dissolving agents. The present invention also provides a method for detecting a fibrinolytic potential in a subject.
Owner:CANADIAN BLOOD SERVICES
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com