Application of 5-(3', 5'-dimethoxybenzylidene)-2-sulfo-imidazole-4-one to preparation of drug for treating cerebrovascular disease
A technology for cerebrovascular diseases and cerebrovascular diseases, applied in cardiovascular system diseases, blood diseases, drug combinations, etc., can solve problems such as toxic side effects, uncertain curative effect, and small clinical curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
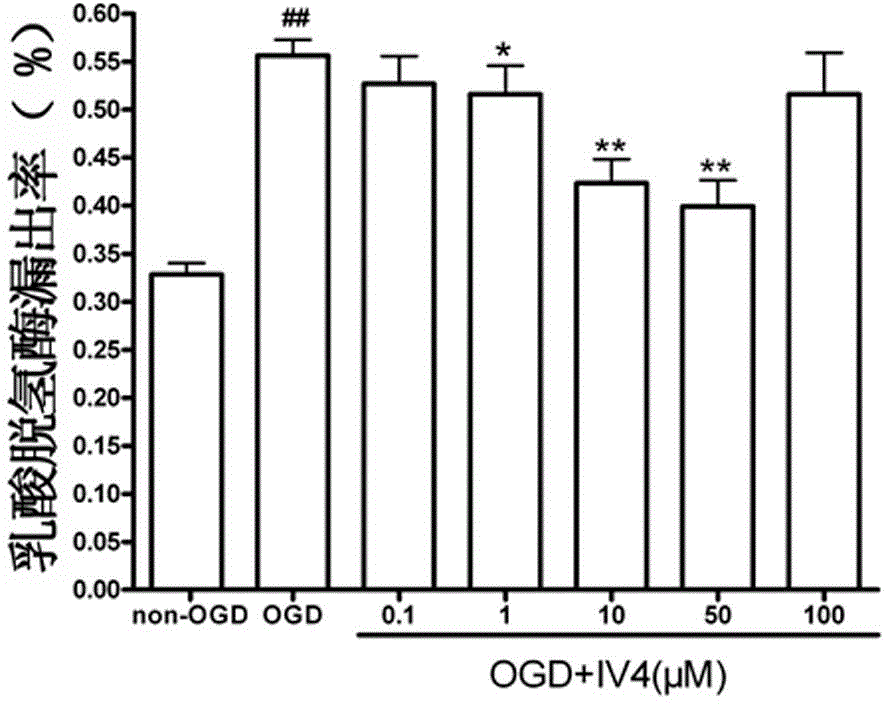
[0025] compound In vitro cultured neural cell line HT 22 Protective effect of cellular glucose and hypoxia injury. This compound is hereinafter referred to as IV 4 .
[0026] Nervous cell line HT 22 The cells were cultured for 24 hours and divided into non-glucose and hypoxia control group, glucose and hypoxia (OGD) group, non-glucose and hypoxia (non-OGD)+IV 4 0.1, 1, 10, 50, 100μM groups and OGD+IV 4 0.1, 1, 10, 50 or 100 μM groups. After glucose and hypoxia for 12 hours, the degree of cell damage was detected by lactate dehydrogenase (LDH) method. According to the formula LDH leakage rate = A culture solution / (A culture solution + A cell homogenate) × 100%.
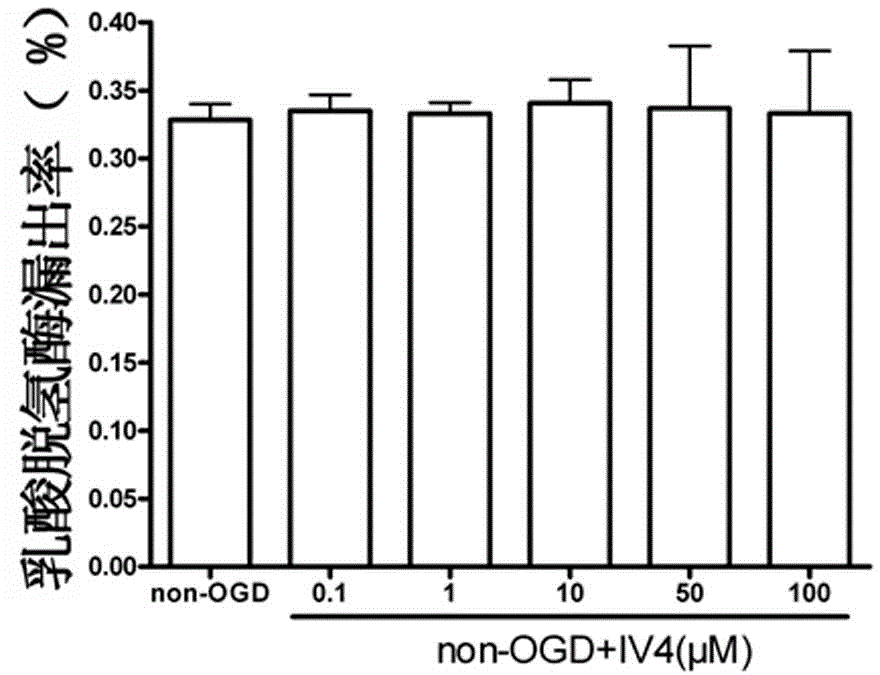
[0027] attached figure 1 Compound IV for single use 4 0.1, 1, 10, 50, 100μM for HT 22 Effect diagram of cell LDH leakage rate, mean ± SD, n=6.
[0028] figure 1 Show that: compared with the control group, IV alone 4 0.1, 1, 10, 50, 100μM had no significant effect on the leakage rate of LDH, indica...
Embodiment 2
[0031] Example 2: Compound IV 4 Protection against permanent focal ischemic brain injury in rats.
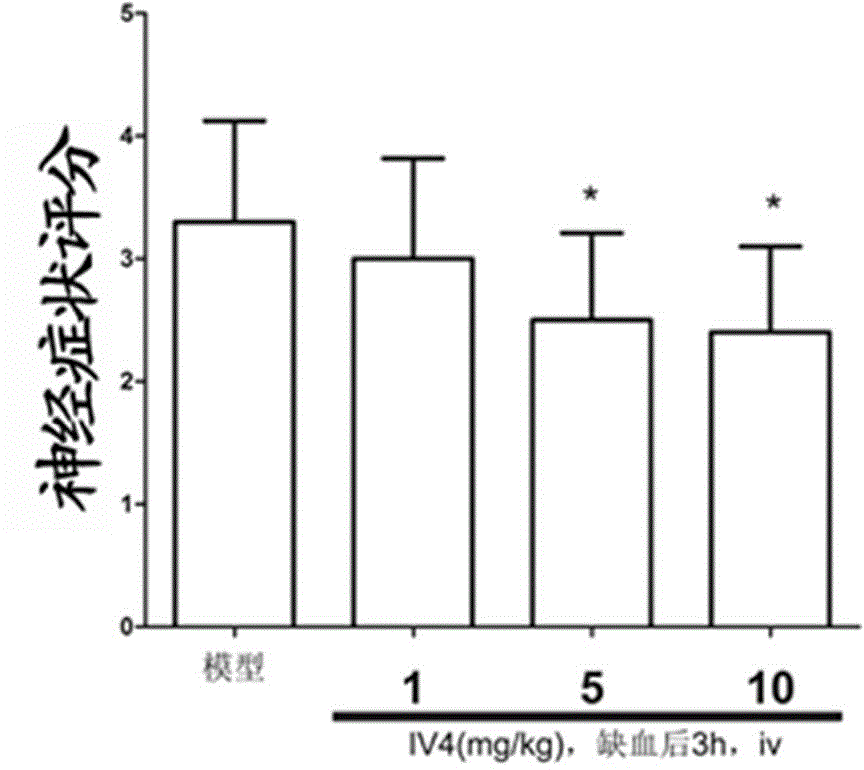
[0032] Male SD rats were randomly divided into control group (sham operation group), model group, IV 4 High, medium and low dose groups (10mg / kg -1 , 5mg / kg -1 and 1mg / kg -1 ), 10 per group. The rat model of permanent middle cerebral artery occlusion (MCAO) was established by suture method. Intravenously administered 3 hours after ischemia 4 , administered in equal volumes at different concentrations. After 24 hours of ischemia, a 5-point scoring method was used. The higher the score, the more obvious the neurological deficit. Nerve function was measured by grasping force method and observed by TTC staining method IV 4 Effect on infarct volume.
[0033] attached image 3 for compound IV 4 Score chart of neurological symptoms of cerebral ischemia rats, mean ± SD, n=10; compared with model group, *p <0.05.
[0034] attached Figure 4 for compound IV 4 Improve the gr...
Embodiment 3
[0037] Example 3: Neural cell line HT 22 The cells were cultured for 24 hours and divided into non-glucose and hypoxia control group (non-OGD group), glucose and hypoxia (OGD) group, non-glucose and hypoxia IV group 4 0.1, 1, 10, 50, 100μM groups and OGD+IV 4 0.1, 1, 10, 50 or 100 μM groups. After 6 hours of glucose and hypoxia, the expression of cathepsin B was detected by Western Blotting.
[0038] attached Figure 6 HT 22 Expression map of cathepsin B in cells. Figure 6 A is a representative Western Blotting diagram; Figure 6 B is a Western Blotting statistical graph; mean±SD, n=3; compared with the control group ## p <0.01; compared with the model group, **p<0.01.
[0039] Figure 6 showed that compared with the non-glucose-hypoxic group, 6 h of glucose-deficient hypoxia significantly induced HT 22 The expression of cathepsin B in cell activity increased; 4 Can significantly inhibit OGD-induced HT 22 Increased expression of cellular active cathepsin B, illus...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com