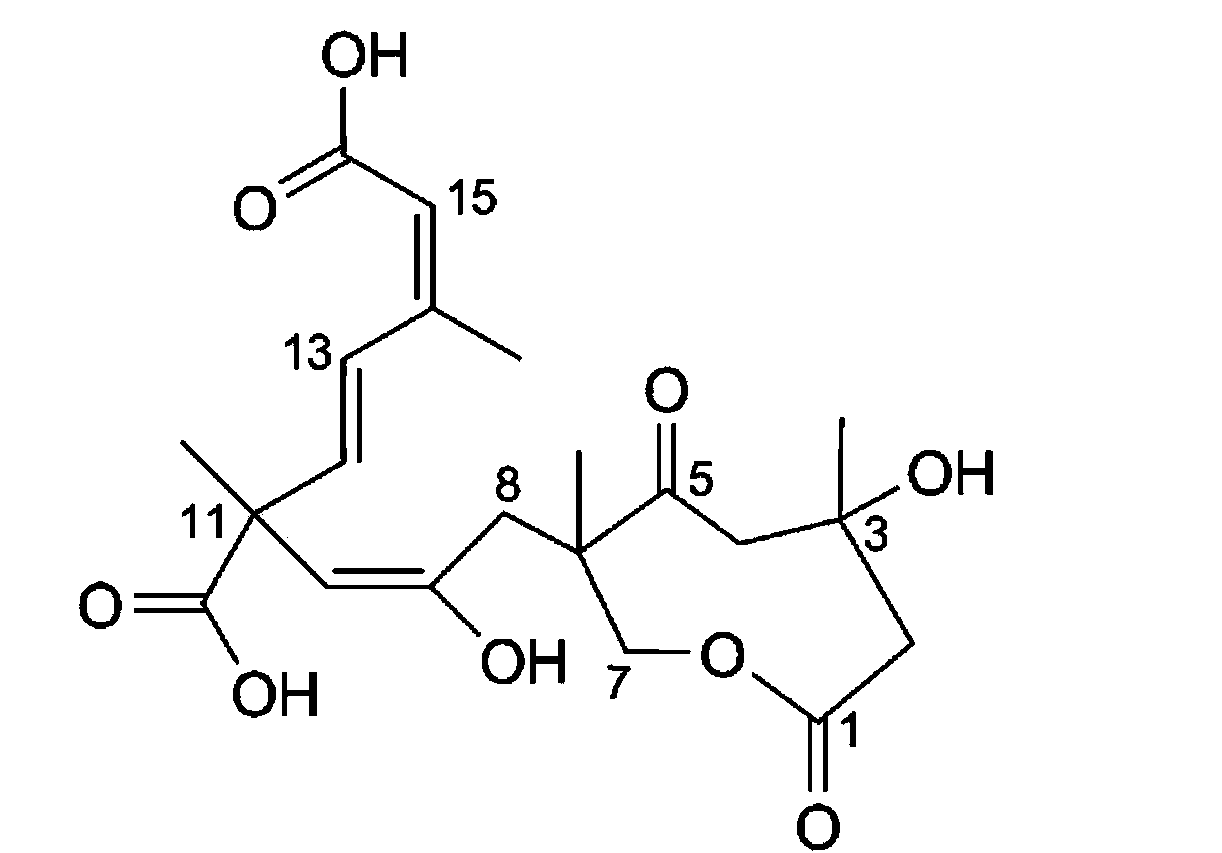
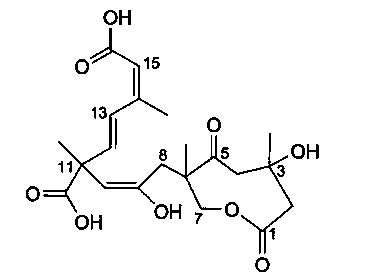
Application of Sarcaboside A to monoamine oxidase inhibitor
A monoamine oxidase and inhibitor technology, which is applied in the field of preparing monoamine oxidase (MAO) inhibitors, can solve problems such as limited application, and achieve the effect of strong inhibitory activity and outstanding substantive characteristics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] Embodiment 1: the preparation of compound Sarcaboside A tablet involved in the present invention:
[0014] Take 20 grams of compound Sarcaboside A, add 180 grams of conventional excipients for tablet preparation, mix well, and make 1000 tablets with a conventional tablet press.
Embodiment 2
[0015] Embodiment 2: the preparation of compound Sarcaboside A capsule involved in the present invention:
[0016] Get 20 grams of compound Sarcaboside A, add conventional adjuvants for preparing capsules such as 180 grams of starch, mix well, and pack into capsules to make 1000 tablets.
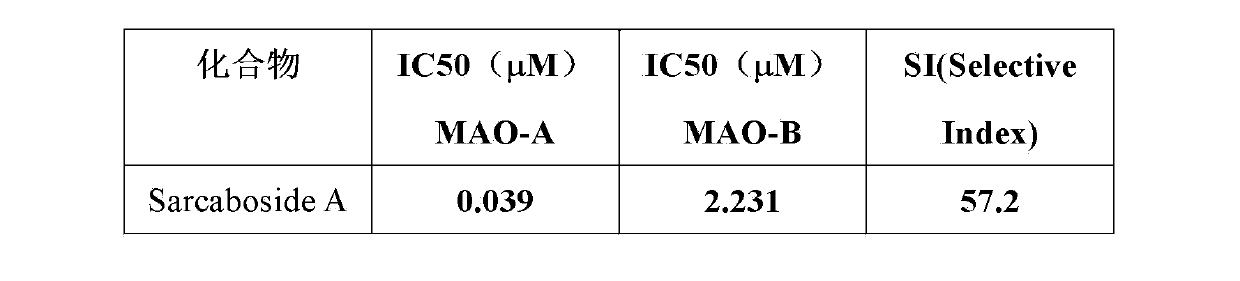
[0017] The following pharmacodynamic experiments will further illustrate its drug activity.
[0018] Compound Sarcaboside A detects the inhibitory activity to MAO-A and MAO-B with the method of fluorescent probe, and described method is carried out according to the following steps:
[0019] Dissolve the sample to be tested in DMSO to prepare a series of samples with concentration gradient. Take 4ul of MAO (10mg / ml), boric acid buffer (PH=8.4), 50ul of BSA (50mg / ml), add the sample solution to be tested, mix well, react in a water bath at 30~38°C for 3h, and then add probe 7 -(3-Aminopropoxy)coumarin 2ul (50mmol / ml), final inhibitor concentration 0~10 -2 mmol / l, the mixture was then reacte...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com