Active targeting star polymer carrier with physiological environment response function and preparation method thereof
A star-shaped polymer and physiological environment technology, which can be applied in the direction of drug combination, anti-tumor drugs, etc., can solve the problems of inability to achieve targeted therapeutic properties of tumor therapy, carrier polymers that are not star-shaped polymers, and inability to achieve carrier stability, etc. , to achieve the effect of easy-to-obtain raw materials, low-cost raw materials, and mature and simple methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
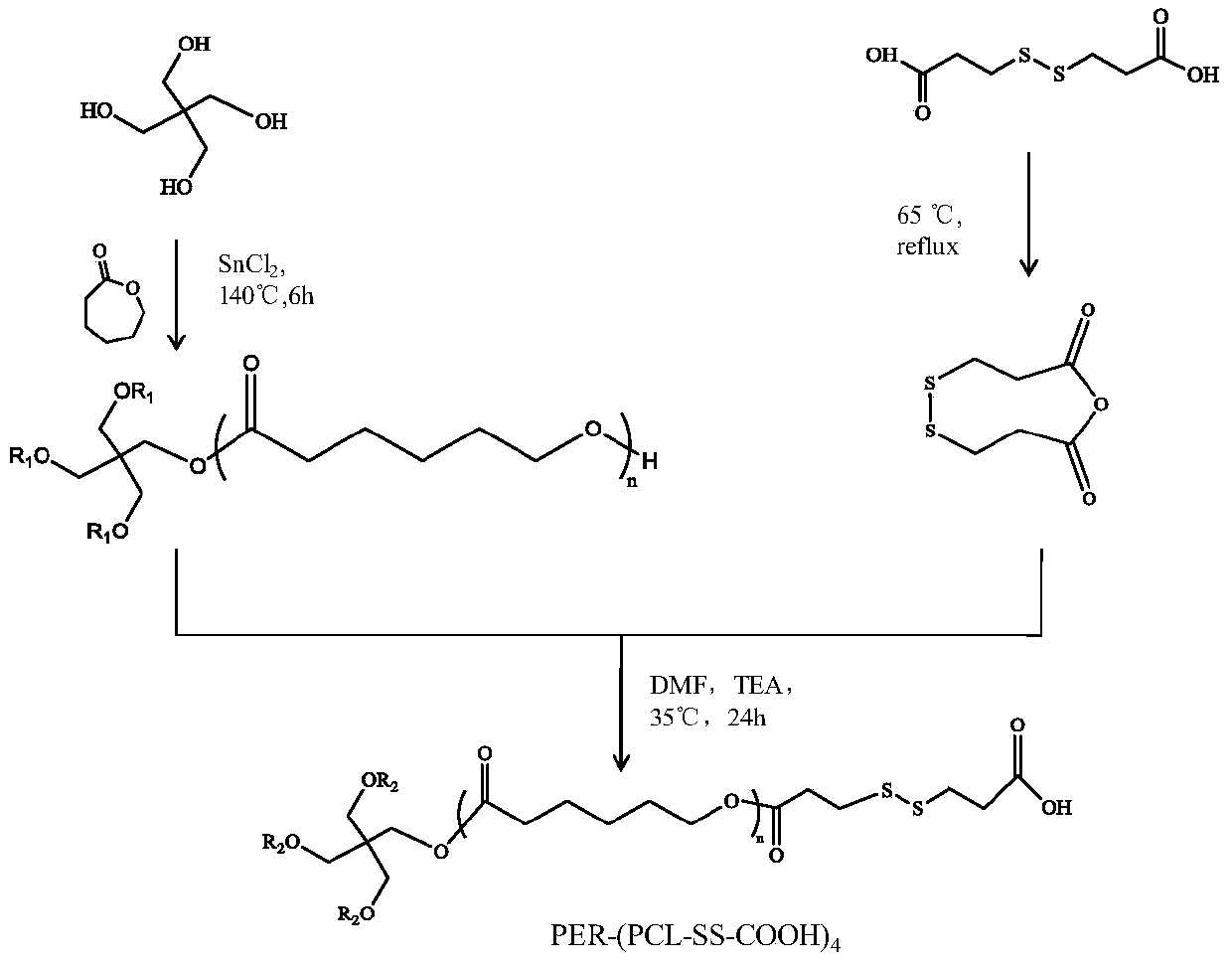
[0020] (1) Preparation of star-shaped polycaprolactone (synthetic route such as figure 1 shown): stannous chloride is used as the catalyst, and the polyol is the nucleus of the star structure. Usually, for 1 equivalent of pentaerythritol, put 70 equivalents of caprolactone and 1% of the total mass of stannous chloride to synthesize star-shaped polycaprolactone with a theoretical molecular weight of 8000. After evacuating for 3 hours, ring-opening polymerization was carried out at a temperature of 150 degrees, and the reaction was carried out for 6 hours. After the product is cooled, it is dissolved in dichloromethane, precipitated in a large amount of ice ethanol, filtered with suction, and the product is vacuum-dried to obtain four-arm polycaprolactone.
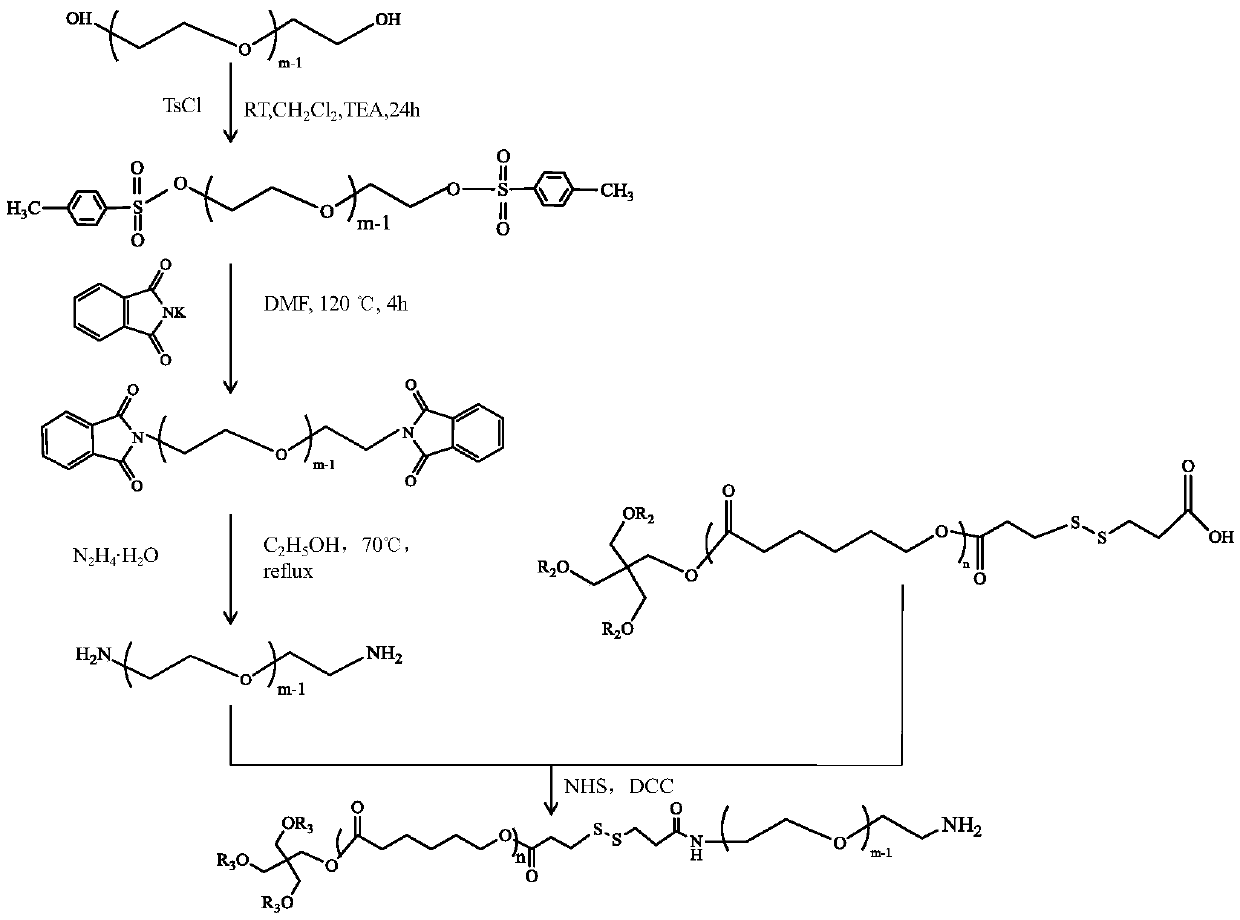
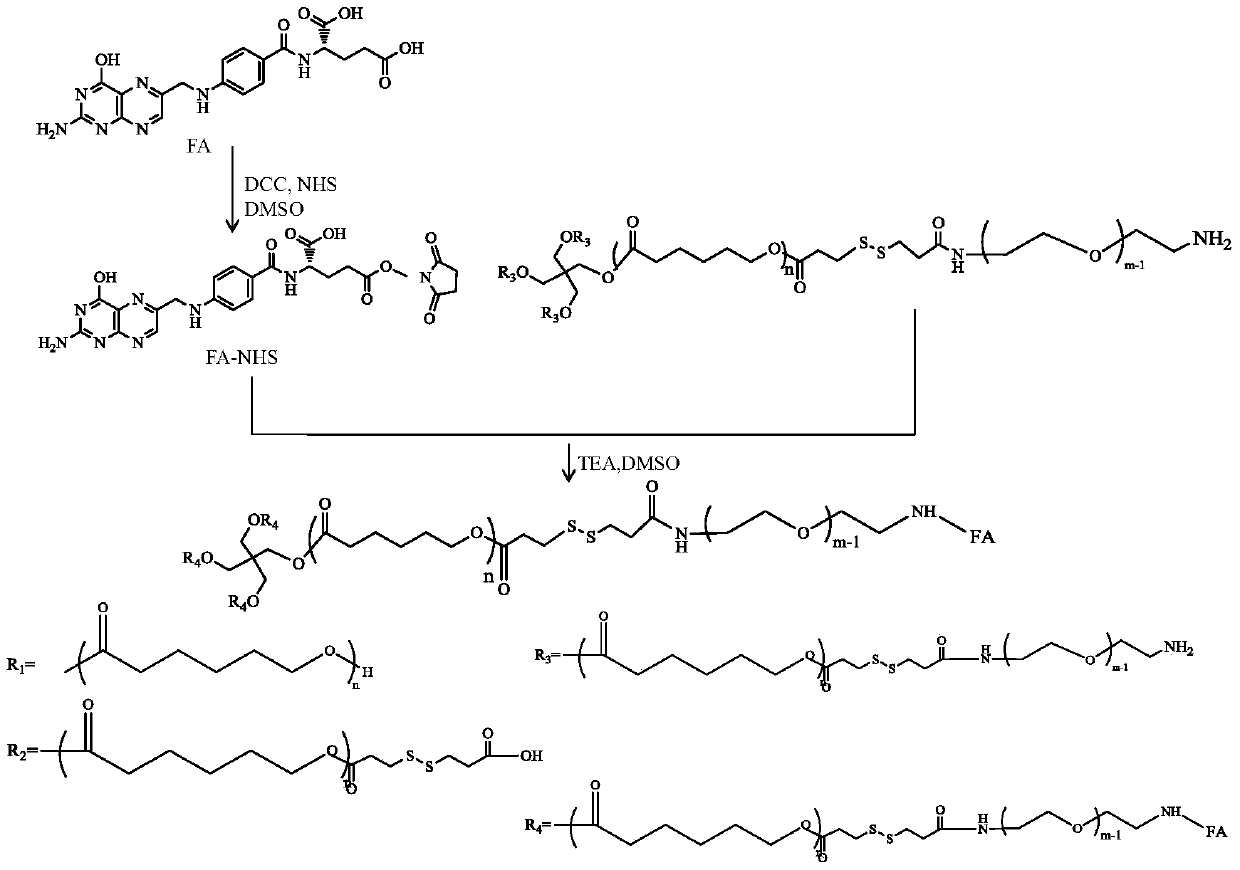
[0021] In the above preparation process, the molecule used as the core of the star structure can be a kind of polyhydric alcohol such as glycerol, pentaerythritol and dipentaerythritol, which respectively form three-arm, fo...
Embodiment 1
[0030]Take by weighing 0.17 gram of pentaerythritol, 10 gram of caprolactone and 0.1 gram of tin protochloride, place in a one-necked bottle, after vacuumizing for 3 hours, ring-opening polymerization under the condition of temperature 150 degrees, take out after 6 hours, treat that the product cools from melting to In solid state, add 5 mL of dichloromethane to dissolve, drop into 250 mL of ice ethanol for precipitation, filter with suction, and dry in vacuum to obtain four-arm polycaprolactone.
[0031] Weigh 2 grams of dithiodipropionic acid, add 20 grams of acetyl chloride, reflux reaction at a temperature of 65 degrees, stir magnetically for 4 hours, remove most of the organic solvent by rotary evaporation, precipitate with glacial ether, filter with suction, and dry in vacuum, namely have to.
[0032] Weigh 1 part of dithiodipropionic anhydride and 5.2 grams of the above-mentioned star-shaped polycaprolactone, add them to 70 mL of DMF, catalyst DMAP 0.3176 grams, triethy...
Embodiment 2
[0036] In this example, the pentaerythritol in Example 1 was replaced with dipentaerythritol, and the ratio of dipentaerythritol to caprolactone was 1:105. Other implementation methods in Example 1 were used to obtain a six-armed star-shaped copolymer.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com