Decellularized vascular matrix and preparation method thereof
A decellularized and vascular technology, which is applied in the field of decellularized vascular matrix and its preparation, can solve the problems of destroying the ultrastructure of ECM, and the mechanical and biological properties of the acellular matrix are difficult to meet the clinical treatment effect, so as to achieve good decellularized effect Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
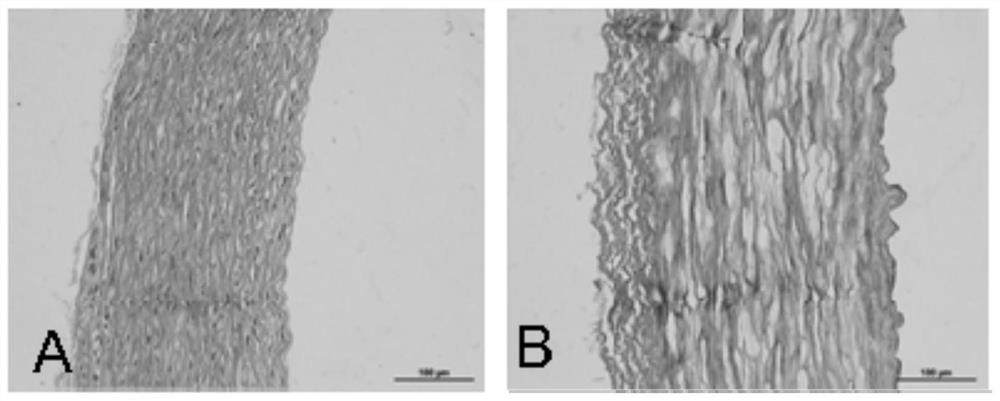
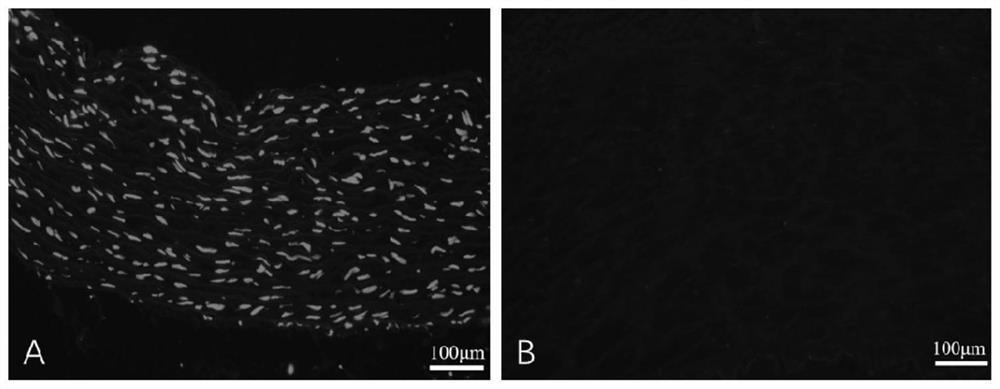
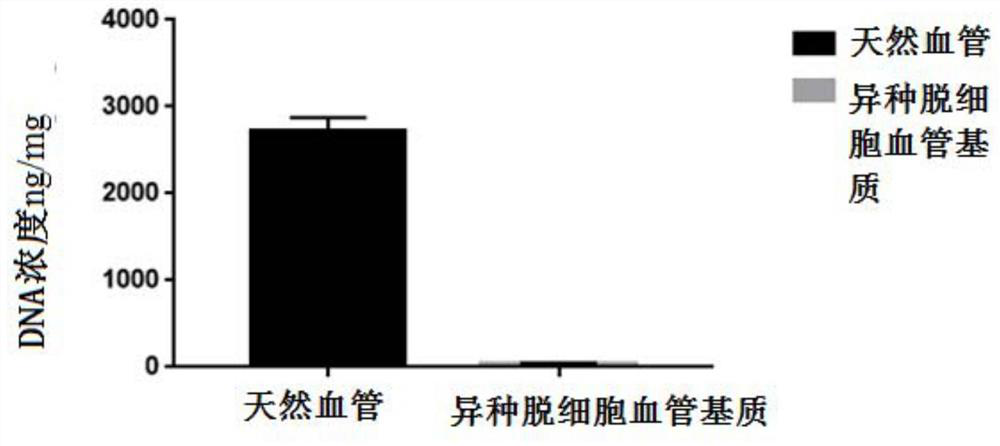
[0043] Example 1 Preparation of heterogeneous decellularized vascular matrix
[0044] This embodiment provides a method for preparing a heterogeneous decellularized vascular matrix, comprising the following steps:
[0045] (1) Pre-treat the vascular tissue to be used, add the vascular tissue into ultrapure water under the ultrasonic conditions of frequency 35KHZ, temperature 41°C, wash twice, 5 minutes each time;
[0046] (2) Dissolve Raptinal in DMSO to make a mother solution containing 20mmol / L Raptinal, and then use MinimumEagle's Medium (MEM) medium to dilute the mother solution containing 20mmol / L Raptinal into a working solution containing 10μmol / L Raptinal , incubate the vascular tissue to be used in the working solution containing 10 μmol / L Raptinal for 24 hours at room temperature (25°C);
[0047] (3) Then add the vascular tissue to be used in step (2) into ultrapure water under the ultrasonic conditions of frequency 35KHZ, temperature 41°C, and wash twice for 5 minu...
Embodiment 2
[0055] Example 2 Preparation of heterogeneous decellularized vascular matrix
[0056] This embodiment provides a method for preparing a heterogeneous decellularized vascular matrix, comprising the following steps:
[0057] (1) Pre-treat the vascular tissue to be used, add the vascular tissue into ultrapure water, the ultrasonic condition is frequency 25KHZ, temperature 45°C, wash 2 times, 5 minutes each time;
[0058] (2) Dissolve Raptinal in DMSO to make a mother solution containing 20mmol / L Raptinal, and then use MinimumEagle's Medium (MEM) medium to dilute the mother solution containing 20mmol / L Raptinal into a working solution containing 10μmol / L Raptinal , incubate the vascular tissue to be used in the working solution containing 10 μmol / L Raptinal for 26 hours at room temperature (25°C);
[0059] (3) Then add the vascular tissue to be used in step (2) into ultrapure water under the ultrasonic condition of frequency 25KHZ, temperature 45°C, wash once, 5 minutes each time...
Embodiment 3
[0067] Example 3 Preparation of heterogeneous decellularized vascular matrix
[0068] This embodiment provides a method for preparing a heterogeneous decellularized vascular matrix, comprising the following steps:
[0069] (1) Pretreat the vascular tissue to be used, and add the vascular tissue into ultrapure water under the ultrasonic condition of frequency 45KHZ, temperature 37°C, wash 3 times, 5 minutes each time;
[0070] (2) Dissolve Raptinal in DMSO to make a mother solution containing 20mmol / L Raptinal, and then use MinimumEagle's Medium (MEM) medium to dilute the mother solution containing 20mmol / L Raptinal into a working solution containing 10μmol / L Raptinal , incubate the vascular tissue to be used in the working solution containing 10 μmol / L Raptinal for 24 hours at room temperature (25°C);
[0071] (3) Then add the vascular tissue to be used in step (2) into ultrapure water under the ultrasonic condition of frequency 45KHZ, temperature 37°C, wash 3 times, 7 minute...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com