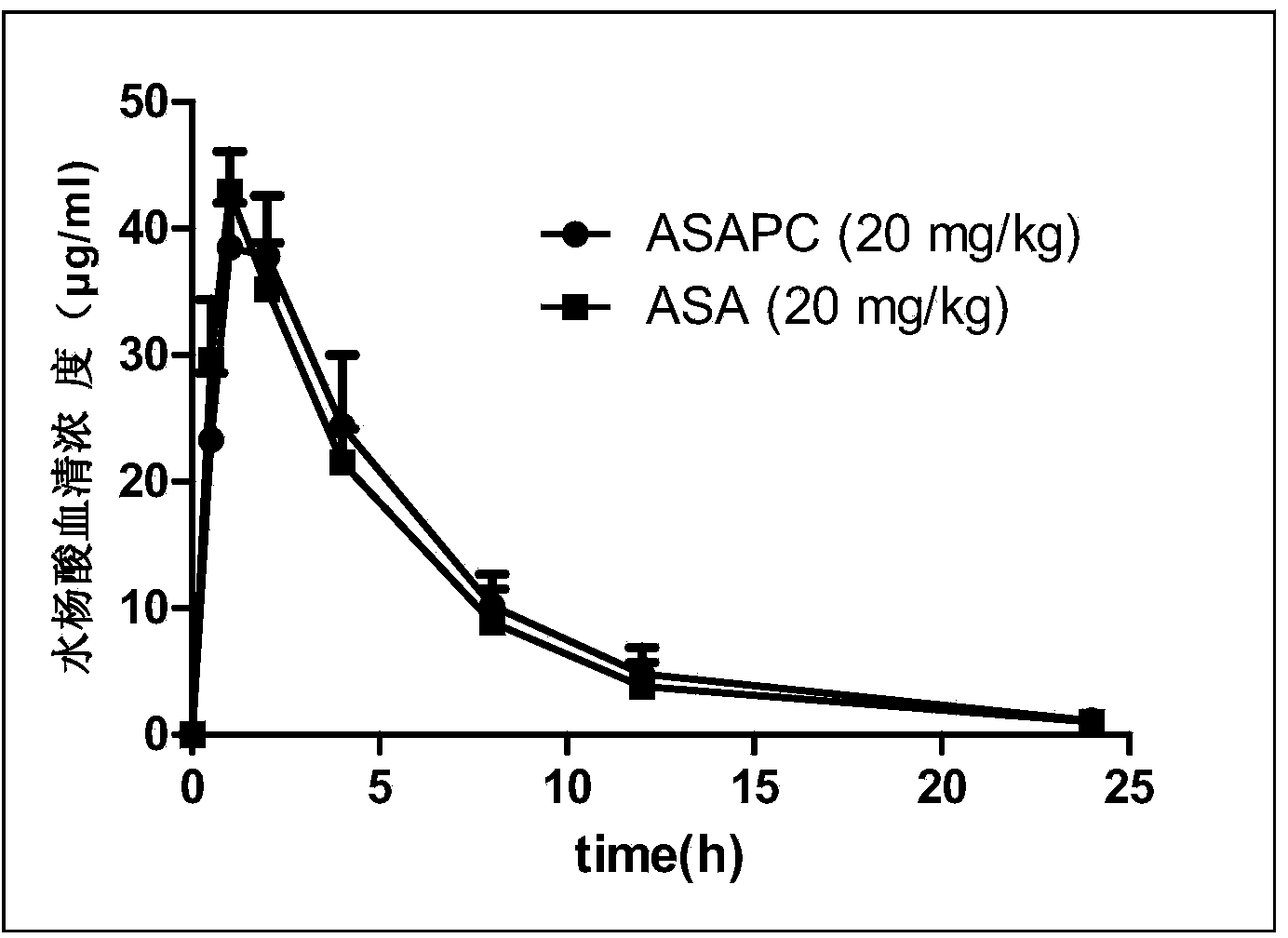
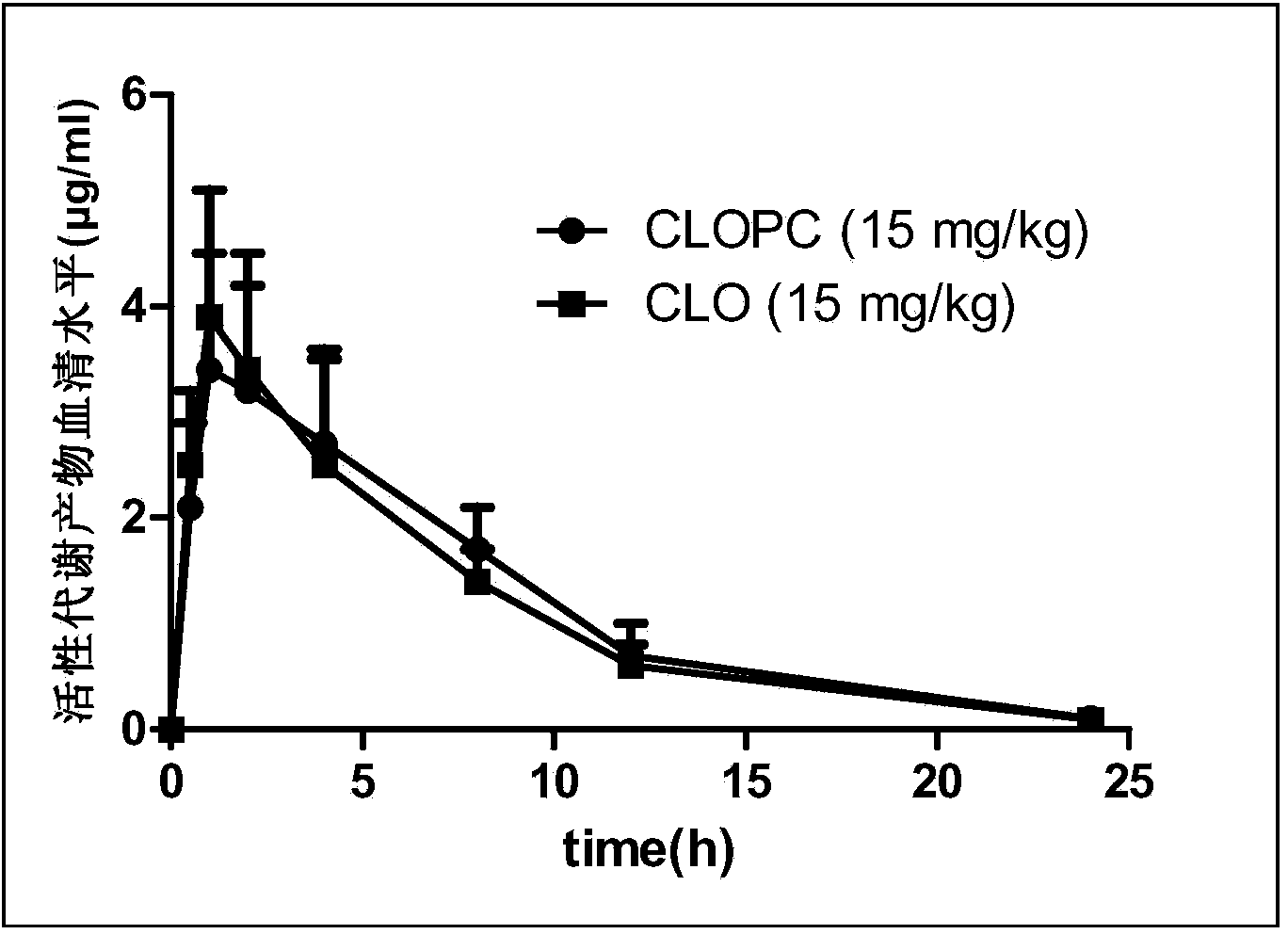
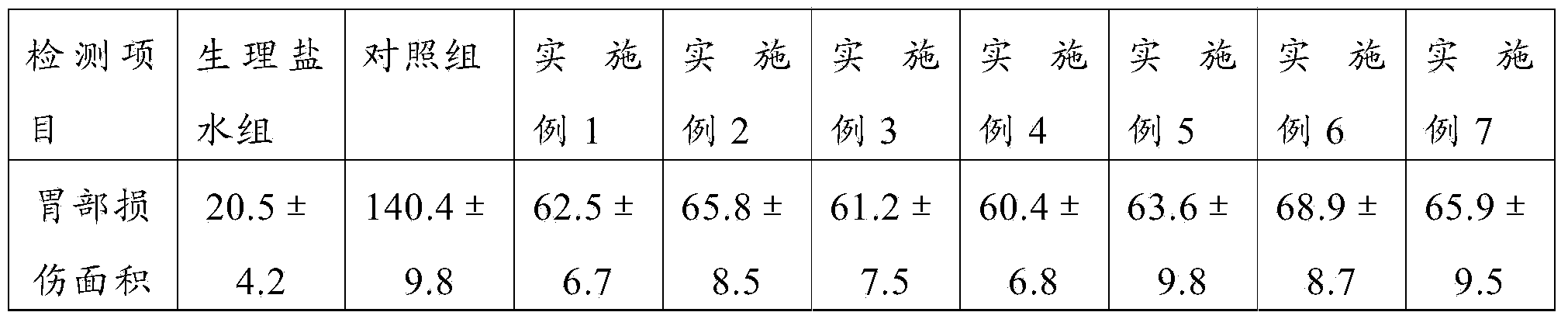
Pharmaceutical composition and preparation method and application thereof
A composition and drug technology, applied in the field of medicine, can solve the problems of use restriction, can not affect the pharmacokinetic level, weaken the efficacy of clopidogrel hydrogen sulfate, etc., and achieve the effect of reducing the risk of gastric injury
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1: Preparation of the pharmaceutical composition of the present invention
[0031] Mix pure ethanol (purity ≥99.5%, 20ml) with soybean lecithin (Phosal35SB, a type of soybean lecithin, 1.75g, phospholipid concentration 37%) in advance at 75°C using a homogenizer; after 5 minutes , add 750 mg of clopidogrel bisulfate and 1.0 g of aspirin, and continue to mix for 15 minutes; then cool to room temperature, and filter with a filter with a pore size of 0.2 microns; finally, lyophilize to remove ethanol to obtain the pharmaceutical composition.
Embodiment 2
[0032] Embodiment 2: Preparation of the pharmaceutical composition of the present invention
[0033] Mix pure ethanol (purity ≥99.5%, 20ml) with soybean lecithin (Phosal35SB, a type of soybean lecithin, 0.875g, phospholipid concentration 20%) in advance at 75°C using a homogenizer; after 5 minutes , add 375 mg of clopidogrel bisulfate and 0.5 g of aspirin, and continue mixing for 15 minutes; then cool to room temperature, and filter with a filter with a pore size of 0.2 microns; finally, lyophilize to remove ethanol to obtain the pharmaceutical composition.
Embodiment 3
[0034] Embodiment 3: Preparation of the pharmaceutical composition of the present invention
[0035] Mix pure ethanol (purity ≥99.5%, 20ml) with soybean lecithin (Phosal35SB, a type of soybean lecithin, 17.5g, phospholipid concentration 10%) in advance at 75°C using a homogenizer; after 5 minutes , add 375 mg of clopidogrel bisulfate and 0.5 g of aspirin, and continue mixing for 15 minutes; then cool to room temperature, and filter with a filter with a pore size of 0.2 microns; finally, lyophilize to remove ethanol to obtain the pharmaceutical composition.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com