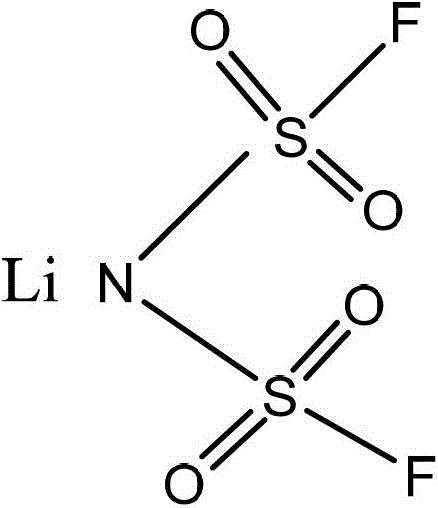
Preparation method of lithium bisfluorosulfonyl imide
A technology of lithium bisfluorosulfonyl imide and lithium bisfluorosulfonyl imide, which is applied in the field of preparation of lithium bisfluorosulfonyl imide, can solve the problems of difficult purification, separation, combustion or explosion of the product, Achieve the effect of easy industrial preparation and simple process route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Under the protection of dry nitrogen, add 219g (1mol) of bisfluorosulfonimide potassium salt, 194g (1mol) of lithium bisoxalate borate, and 1000ml of dimethyl carbonate into a three-necked flask. Stir and react at room temperature for 12 hours. Filter under reduced pressure to remove insoluble After concentrating the filtrate to about 150 ml under reduced pressure, an equal volume of dichloromethane is added, and the mixture is cooled to room temperature and filtered, washed, and dried to obtain 150 g of lithium bisfluorosulfonimide.
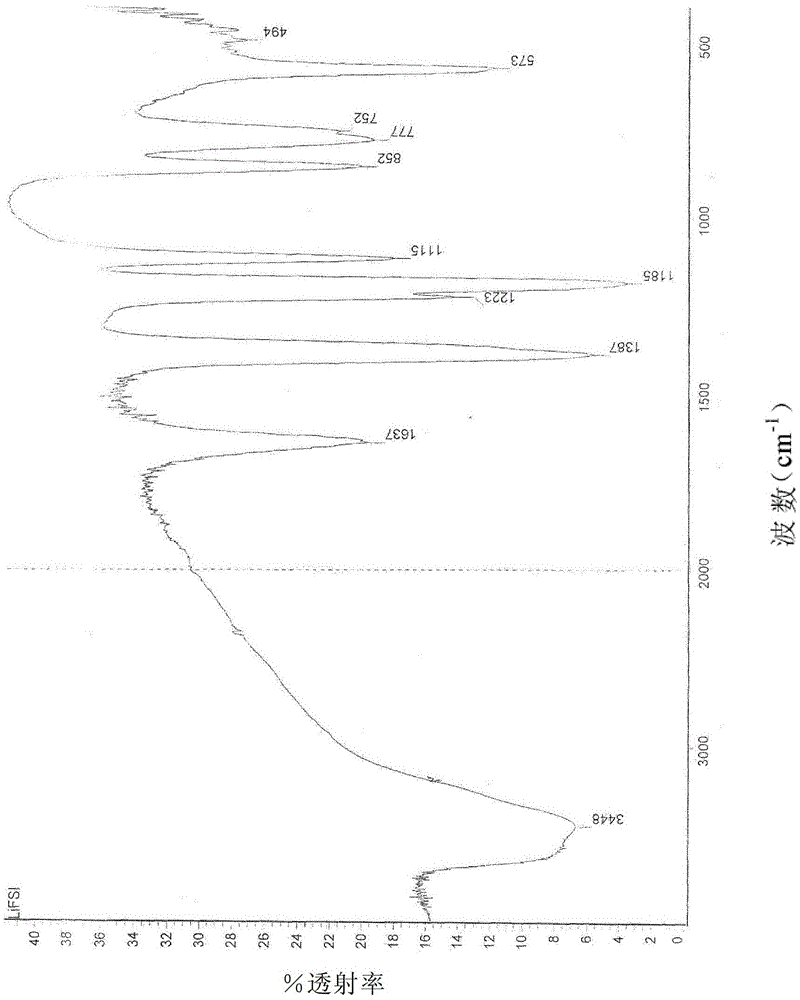
[0032] The product has been inspected by infrared, at 1387cm -1 ,1223cm -1 ,1185cm -1 ,852cm -1 ,777cm -1 , 573cm -1 By comparing the standard spectra of bisfluorosulfonimide, it is determined as the characteristic group of bisfluorosulfonimide lithium.
Embodiment 2
[0034] Under the protection of dry nitrogen, add 219g (1mol) of bisfluorosulfonimide potassium salt, 144g (1mol) of lithium oxalate difluoroborate and 1000ml of dimethyl carbonate into a three-necked flask, stir and react at room temperature for 12 hours, and remove by filtration under reduced pressure. Insoluble matter, the filtrate was concentrated to about 150ml under reduced pressure, and then an equal volume of dichloromethane was added. After cooling to room temperature, it was filtered, washed and dried to obtain 138g of lithium bisfluorosulfonimide.
[0035] The product has been inspected by infrared, at 1387cm -1 ,1223cm -1 ,1185cm -1 ,852cm -1 ,777cm -1 ,573cm -1 By comparing the standard spectra of bisfluorosulfonimide, it is determined as the characteristic group of bisfluorosulfonimide lithium.
Embodiment 3
[0037] Add 219g (1mol) of bisfluorosulfonimide potassium salt, 194g (1mol) of lithium bisoxalate borate and 1200ml of ethyl methyl carbonate to a three-necked flask under the protection of dry nitrogen. Stir and react at room temperature for 12 hours. Filter under reduced pressure to remove insoluble After the filtrate was concentrated to about 165ml under reduced pressure, an equal volume of dichloromethane was added, and after it was cooled to room temperature, it was filtered, washed, and dried to obtain 158g of lithium bisfluorosulfonimide.
[0038] The product has been inspected by infrared, at 1387cm -1 ,1223cm -1 ,1185cm -1 ,852cm -1 ,777cm -1 , 573cm -1 By comparing the standard spectra of bisfluorosulfonimide, it is determined as the characteristic group of bisfluorosulfonimide lithium.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com