Congenital aniridia disease-causing gene, kit for detecting congenital aniridia disease-causing gene and application of gene
A disease-causing gene and aniridia technology, applied in application, genetic engineering, plant genetic improvement, etc., can solve problems that can only be seen under gonioscopy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Example 1 Preparation of a kit for detection of congenital aniridia disease-causing genes
[0054] 1. Design and synthesis of primer pairs
[0055] Primer 1: CGTAAGCTTGTCATTGTTTAATGC (shown in SEQ ID NO: 1)
[0056] Primer 2: AGAGAGGGTGGGAGGAGGTA (shown in SEQ ID NO: 2);
[0057] It was synthesized by an automatic DNA synthesizer and diluted to 20 μmol / L.
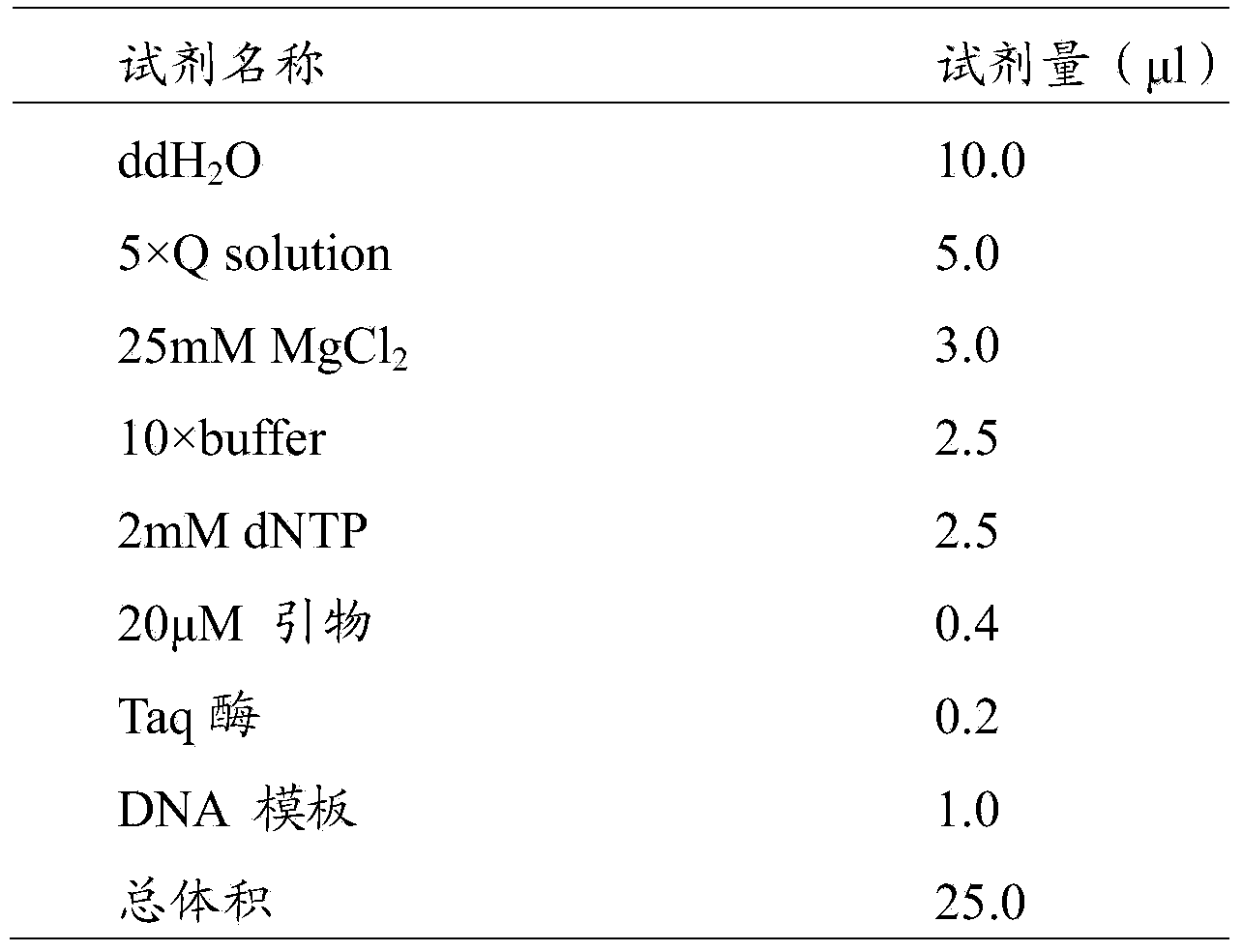
[0058] 2. Assembling the kit
[0059] Including: 2mM dNTP, 10×PCR reaction buffer, DNA polymerase, PCR amplification primer pair and double distilled water.
Embodiment 2
[0060] Example 2 Application of a kit for detection of congenital aniridia pathogenic gene in clinical detection
[0061] 1. Test samples and experimental materials:
[0062] Experimental materials: Genomic DNA extraction kit QIAamp DNA Blood Mini Kit was purchased from QIAGEN; PCR detection kit was purchased from Qiagen, Hilden, Germany.
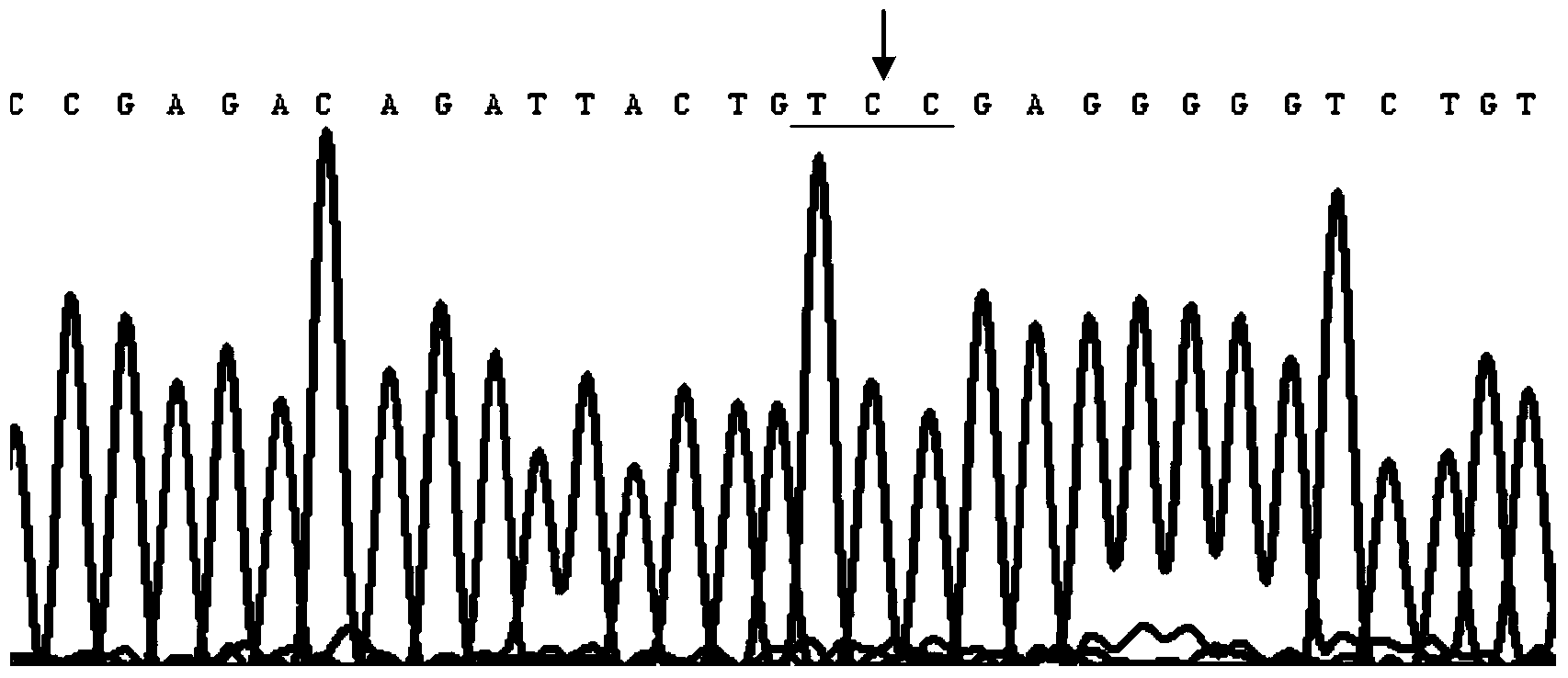
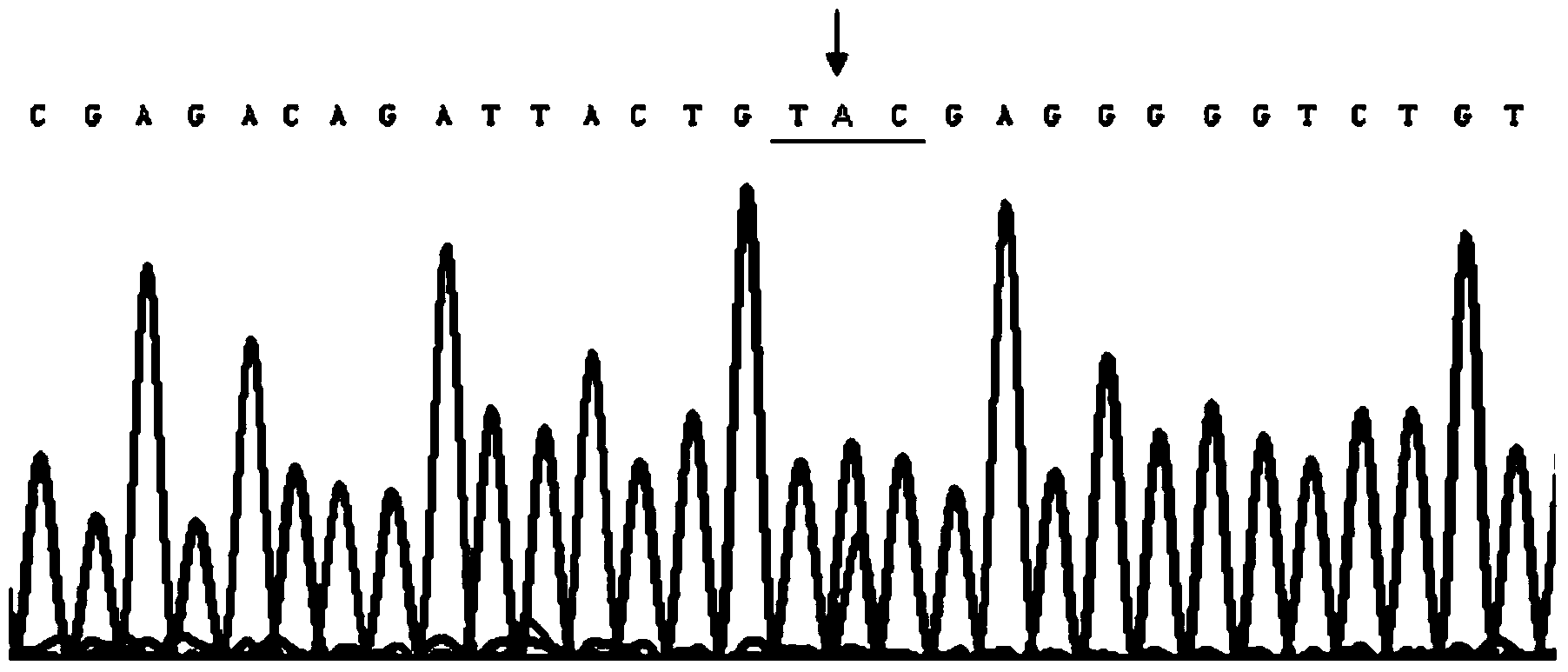
[0063] Test samples: A family with congenital aniridia was collected clinically. Family members were investigated and visited or had a general examination to exclude other diseases and systemic abnormalities. Detailed ophthalmic examinations, including visual acuity, slit-lamp microscopy, and dilated fundus examinations, were performed on family members. All patients were photographed under a slit lamp microscope. All family members who participated in the investigation and were blood samples were understood the purpose and meaning of this study and signed the informed consent. There were 4 generations in this family, 36 family members,...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap