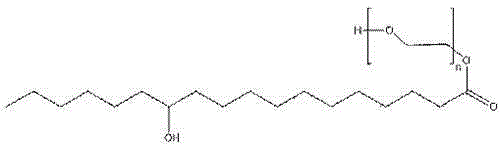
A kind of synthetic method of polyethylene glycol-12-hydroxystearate
A technology of hydroxystearate and hydroxystearic acid, applied in the field of synthesis of polyethylene glycol-12-hydroxystearate, can solve the problems of difficult control, narrow molecular weight distribution, wide molecular weight distribution and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
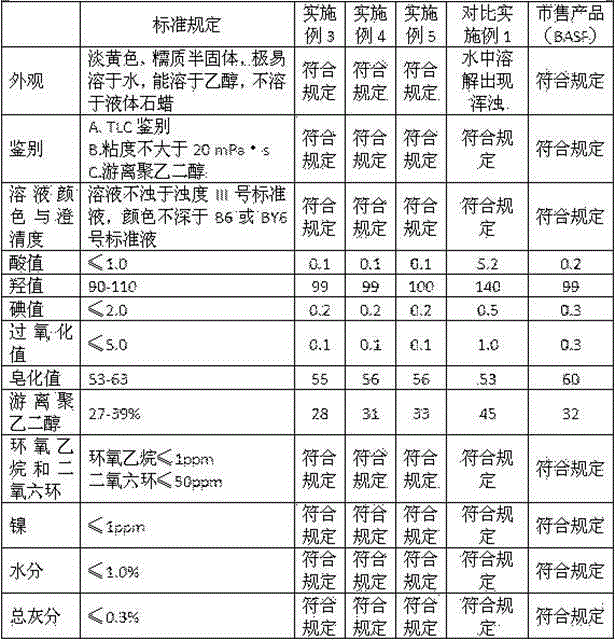
Image
Examples
Embodiment 1
[0044] Example 1 Preparation of selective hydroxyl protecting agent tris (benzhydryl) phosphate
[0045] raw material:
[0046] Benzophenone hydrazone (CAS No.: 5350-57-2), purchased from Bailingwei, with a purity of 95%;
[0047] Iodobenzenediacetic acid (CAS No.: 3240-34-4), purchased from Bailingwei, with a purity of 98%;
[0048] 85% phosphoric acid, dichloromethane, sodium chloride, and ethyl acetate are commercially available analytical reagents.
[0049] The above reagents were used directly without any pretreatment.
[0050] step:
[0051] (a) Put 58.8g (0.3mol) of benzophenone hydrazone and 11.5g (0.1mol) of 85% phosphoric acid into 500ml of dichloromethane solvent, add 3.2g (0.01mol) of iodobenzenediacetic acid as a catalyst, at 40 ℃ reflux reaction for 4h;
[0052] (b) After the reaction, wash with saturated NaCl solution, 100ml each time until the washing solution is neutral, combine the organic layers, dry with anhydrous sodium sulfate for 2 hours, evaporate ...
Embodiment 2
[0054] Example 2 Preparation of Ethoxylation Reaction Composite Catalyst
[0055] Put 15.8g of magnesium acetate solid and 17.4g of potassium hydroxide solid into the mortar, grind for 15 minutes and mix them evenly, then add 17.1g of barium sulfate powder in batches under the grinding state, and continue grinding after adding the barium hydroxide solid 30 min, and then vacuum-dried at 70° C. for 2 h to obtain a catalyst.
Embodiment 3
[0056] Embodiment 3 Preparation of polyethylene glycol-12-hydroxystearate
[0057] raw material:
[0058] 12-Hydroxystearic acid (CAS No.: 106-14-9), purchased from Qingdao Tongkai Castor Chemical Co., Ltd., premium product.
[0059] Ethylene oxide and other inorganic and organic chemical reagents are commercially available industrial grade or analytically pure products.
[0060] The operation method is as follows:
[0061] First, it is the pretreatment step of hydroxystearic acid, that is, the step of protecting the hydroxyl group of 12-hydroxystearic acid.
[0062] 1) Take 30.0g (0.1mol) 12-hydroxystearic acid, add appropriate amount of CH 2 Cl 2 The solvent is made into a solution, and a selective hydroxyl protecting agent (Ph 2 CHO) 3 PO 61.4g (0.103mol), and 0.57g (0.005mol) trifluoroacetic acid catalyst, heated to reflux for 2-4h;
[0063] 2) Wash the reaction solution with purified water and saturated NaCl solution until the washing solution is neutral, combine t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com