Pyrrolobenzodiazepines and conjugates thereof
一种偶联物、药物的技术,应用在药物组合、医药配方、有机化学等方向,能够解决失活活性、细胞毒性药物易等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
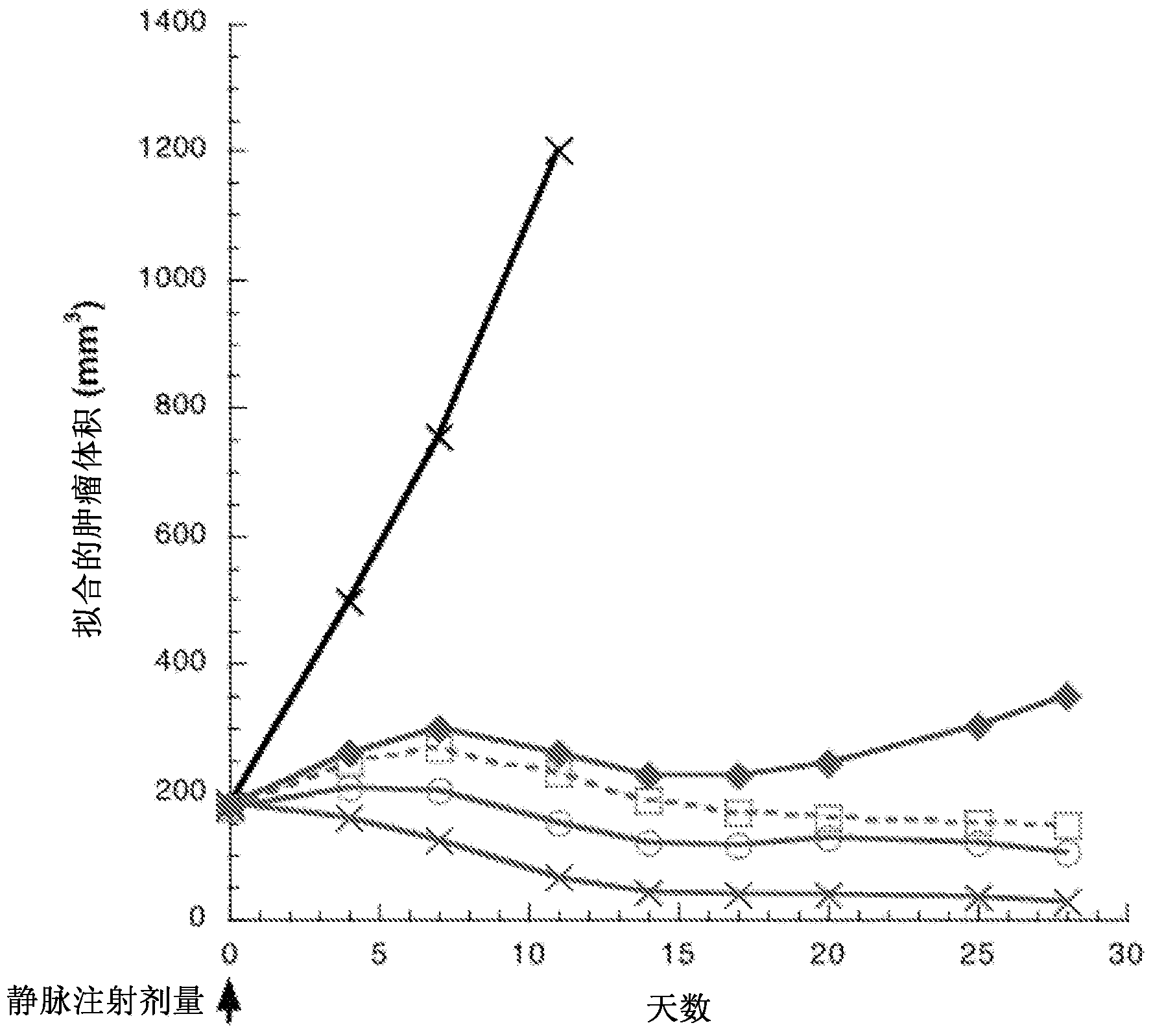
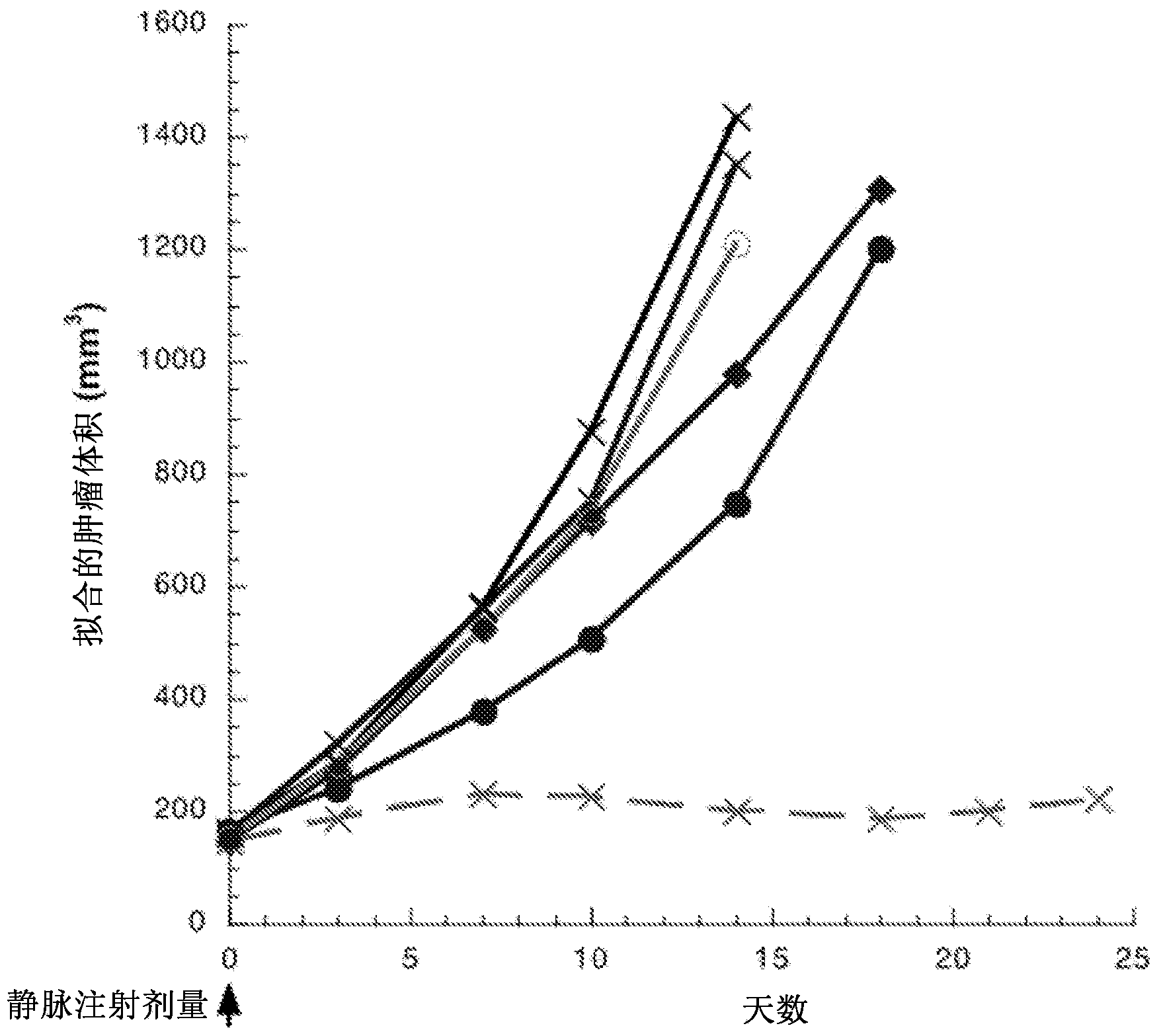
[0570] Preparation of Antibody Drug Conjugates
[0571] Antibody drug conjugates can be prepared by several routes using those organic chemical reactions, conditions and reagents well known to those skilled in the art, including: (1) reacting the nucleophilic group of the antibody with a divalent linker reagent to pass covalently bonding to form the antibody-linker intermediate Ab-L, followed by reaction with the activated drug moiety reagent; and (2) reacting the drug moiety reagent with the linker reagent to covalently form the drug-linker reagent D-L, which is then reacted with the antibody nucleophilic group reaction. Conjugation Methods (1) and (2) Various antibodies and linkers can be employed to prepare the antibody-drug conjugates of the present invention.
[0572] Nucleophilic groups of antibodies include, but are not limited to, side chain sulfhydryl groups such as cysteine. Thiol groups are nucleophilic and are capable of reacting with electrophilic groups on the ...
Embodiment 1
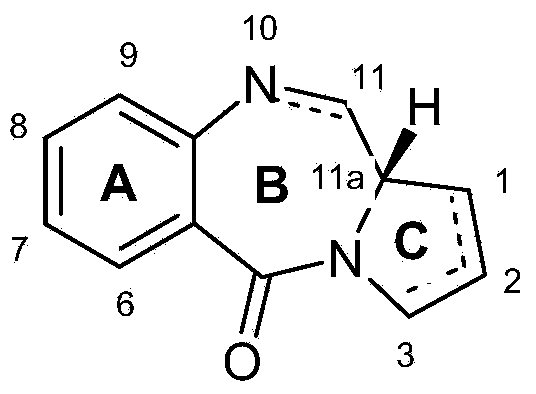
[0599] (a) (S)-2-(methoxycarbonyl)-4-methylenepyrrolidinium chloride (3)
[0600]
[0601] (i) (S)-1-tert-Butyl 2-methyl 4-methylenepyrrolidine-1,2-dicarboxylate (2)
[0602] Potassium carbonate (19.92 g, 14 mmol, 3 eq.) was added to a stirred solution of carboxylic acid (1) (10.92 g, 48 mmol, 1 eq.) in DMF (270 mL). The resulting white suspension was stirred at room temperature for 30 min, at which time iodomethane (21.48 g, 9.5 mL, 151 mmol, 3.15 eq.) was added. The reaction mixture was stirred at room temperature for 3 days. DMF was removed by rotary evaporation under reduced pressure to give a yellow residue which was partitioned between ethyl acetate and water. The organic phase was separated and the aqueous phase was extracted with ethyl acetate. The combined organic layers were washed with water, brine and dried over magnesium sulfate. Ethyl acetate was removed by rotary evaporation under reduced pressure to yield the crude product as a yellow oil. The crude pro...
Embodiment 2
[0630] (a) (R)-2-(pyridin-2-yldisulfanyl)propan-1-ol (18)
[0631]
[0632] (i) (R)-Methyl 2-(acetylthio)propionate (16)
[0633] Thioacetic acid (1.99 g, 1.86 mL, 26.1 mmol, 1.1 eq.) was added to a suspension of cesium carbonate (7.73 g, 23.72 mmol, 1.0 eq.) in dry DMF (40 mL). After 30 minutes, (S)-methyl 2-chloropropionate (15) was added and the mixture was stirred at room temperature for 1 hour. The reaction mixture was partitioned between diethyl ether (150 mL) and water (150 mL); the water was separated and washed with another portion of diethyl ether (150 mL). The combined organic fractions were washed with water (6×100 mL), brine (200 mL), dried (MgSO 4 ) and evaporated under reduced pressure. Purification by flash column chromatography [10% ethyl acetate / 90% n-hexane] gave the product as a colorless oil (3.01 g, 82%). Analytical data: RT 2.25 min; MS (ES + ) m / z (relative intensity) 163 ([M+H] +. ,10),185([M+Na] +. ,65); [α] t d =[+141] 17.8℃ d (c, 2.26C...
PUM
| Property | Measurement | Unit |
|---|---|---|
| composition ratio | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com