Application of adenosine compound in preparation of drugs for prevention and treatment of stress disorders
An adenosine compound, stress disorder technology, applied in the field of medicine, can solve the problems of decreased learning and memory ability, stress disorder and related diseases that have not been seen in WS070133 and similar structural compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
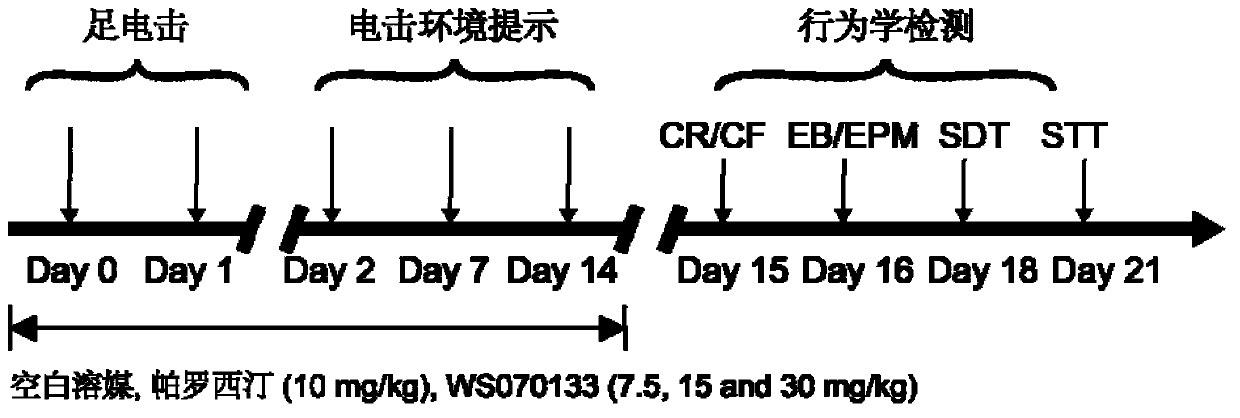
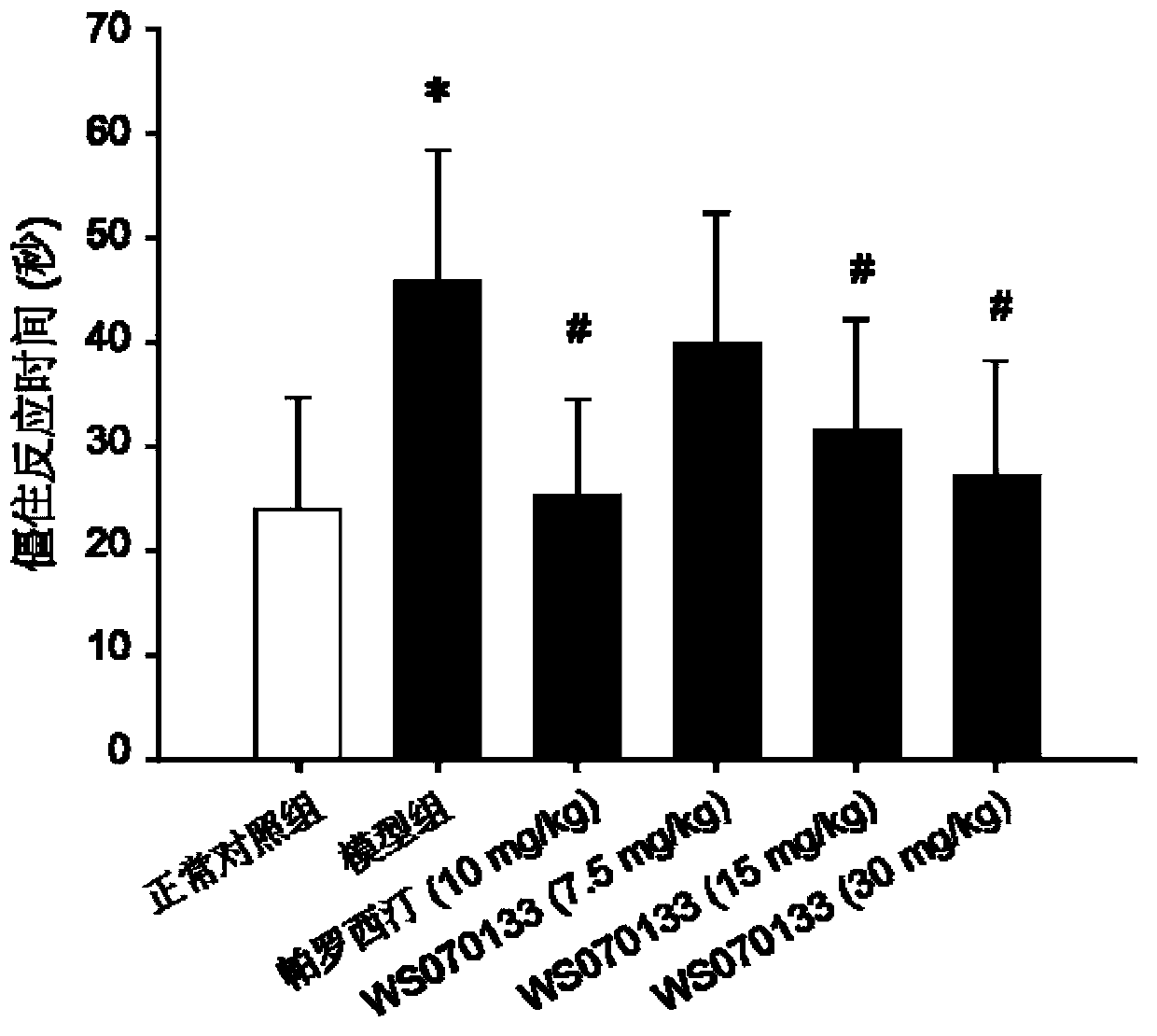
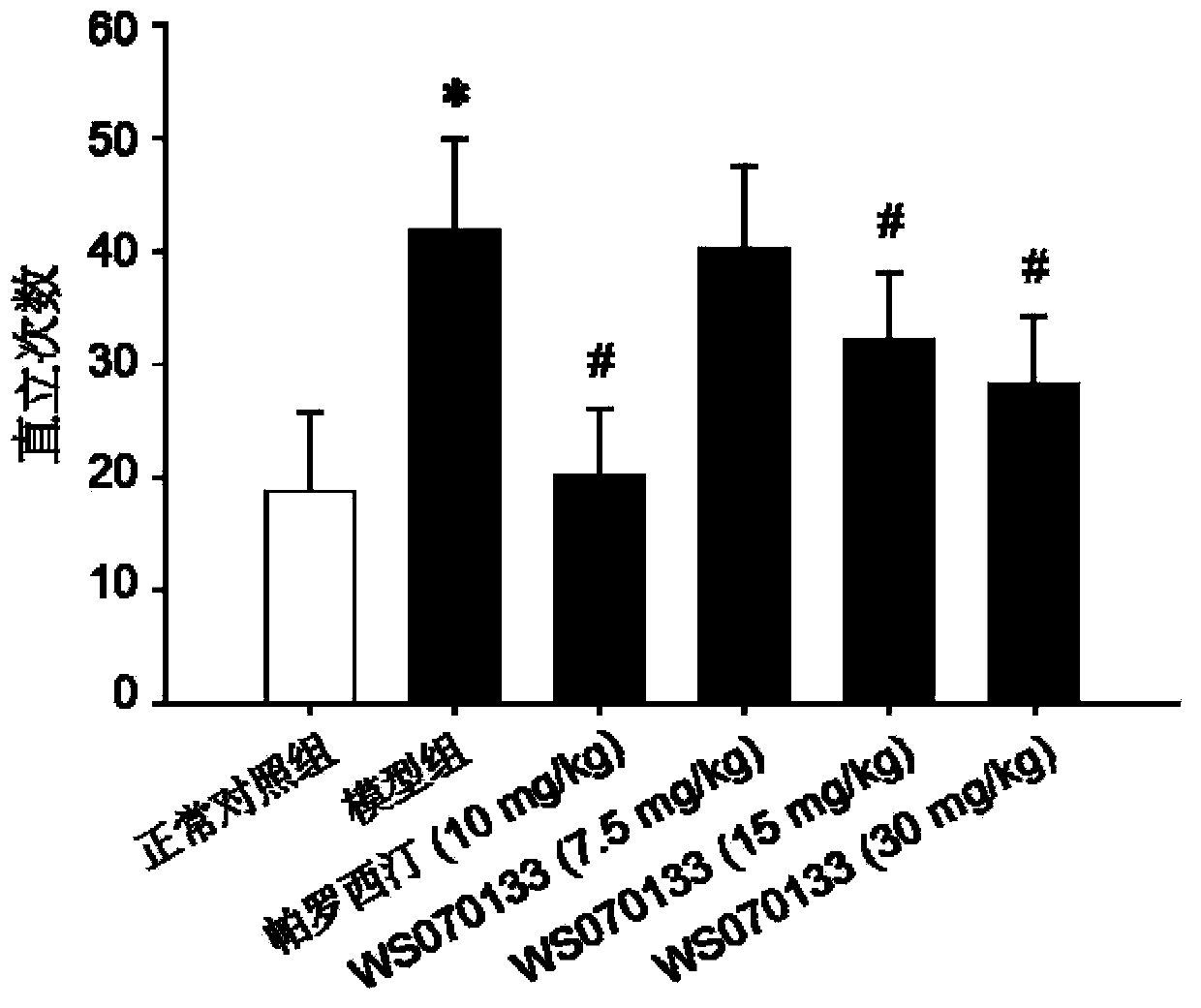
[0036] Example 1: Effect of WS070133 in PTSD Mouse Conditioned Electric Shock Model
[0037] 1. Main method
[0038] 1. Experimental device:
[0039] Mouse elevated plus maze (developed by Institute of Materia Medica, Chinese Academy of Medical Sciences).
[0040] Mouse opening device (Shanghai Yishu Information Technology Co., Ltd.).
[0041] YLS-9A Physiological and Pharmacological Electronic Stimulator (Shandong Jinan Yiyan Technology Development Co., Ltd.)
[0042] Mouse electric shock box (developed by Institute of Materia Medica, Chinese Academy of Medical Sciences)
[0043] Mouse jumping box / avoidance box (developed by Institute of Materia Medica, Chinese Academy of Medical Sciences)
[0044] 2. Experimental animals:
[0045] 60 SPF-grade ICR healthy male mice were provided by Beijing Weitong Lihua Experimental Animal Technology Co., Ltd., and the animal certificate number was SCXK (Beijing) 2009-0007. The initial weight is 12-14g, 10 in each group. Room temperat...
Embodiment 2
[0067] Example 2: Effect of WS070133 in PTSD mouse compound stress model
[0068] 1. Main method
[0069] 1. Experimental device:
[0070] Mouse elevated plus maze (developed by Institute of Materia Medica, Chinese Academy of Medical Sciences).
[0071] Mouse opening device (Shanghai Yishu Information Technology Co., Ltd.).
[0072] YLS-9A Physiological and Pharmacological Electronic Stimulator (Shandong Jinan Yiyan Technology Development Co., Ltd.)
[0073] Mouse electric shock box (developed by Institute of Materia Medica, Chinese Academy of Medical Sciences).
[0074] Mouse water maze (developed by Institute of Materia Medica, Chinese Academy of Medical Sciences)
[0075] Mouse fixation cylinder (Shandong Jinan Yiyan Technology Development Co., Ltd.)
[0076] 2. Experimental animals:
[0077] Seventy-two clean-grade Kunming male mice were provided by Beijing Huafukang Biotechnology Co., Ltd., license number: SCXK (Beijing) 2009-0007. The initial weight is 12-14g, 12 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com