A kind of synthetic technique of oxadiazone
A synthesis process and technology of oxadiazon, applied in the field of organic compound synthesis, can solve problems such as phosgene poisoning, and achieve the effect of avoiding potential safety hazards and high safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
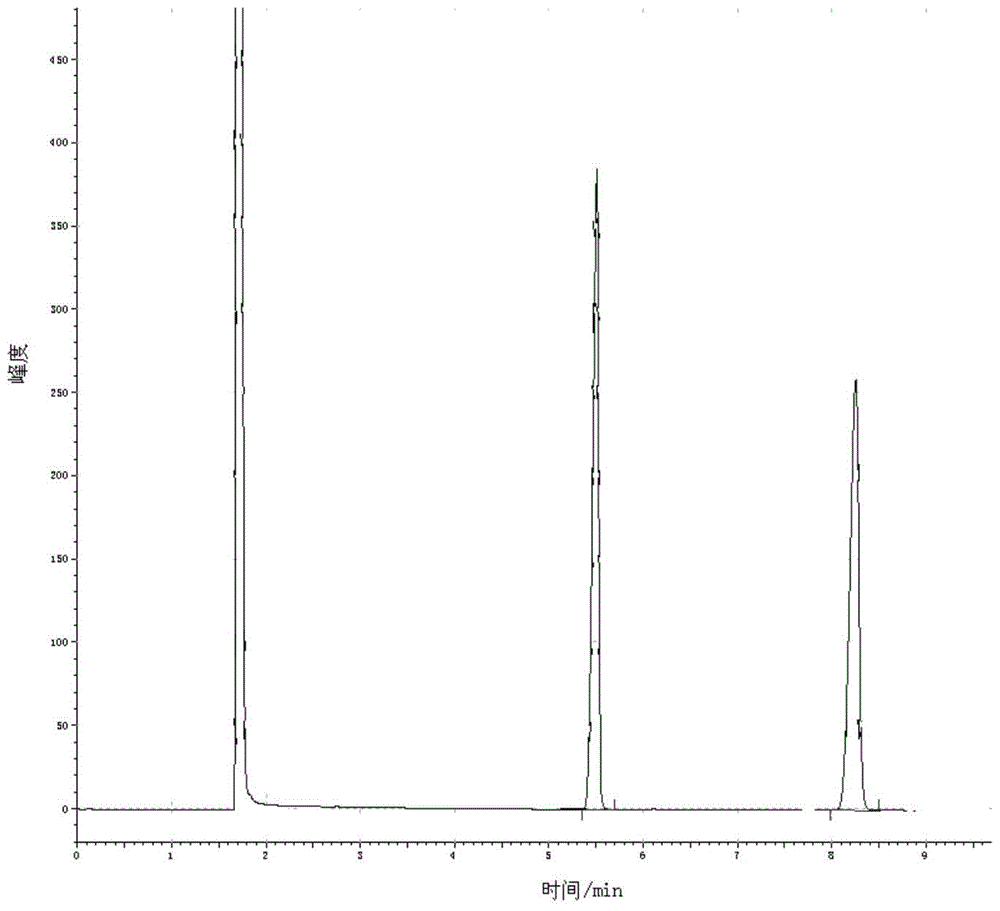
[0044]Add 5.0Kmol of 2,4-dichloro-5-isopropoxyphenylhydrazide and an appropriate amount of toluene solution into the reaction kettle, stir and dissolve, slowly add 5.3Kmol of methyl chloroformate solution dropwise, and the dropwise addition is completed within 2 hours. Insulate for 2 hours, slowly raise the temperature to reflux, reflux for 3 hours, take a sample to detect that the residual hydrazide is 0.1%, which is regarded as the end of the reaction, add catalyst anhydrous sodium methoxide 0.05Kmol, keep the temperature at 90-110°C for 6 hours, and the by-product methanol After the reaction is completed, the temperature is lowered, washed with water, crystallized, and dried to obtain 1712.2 kg of the oxadiazone product. It is detected by gas chromatography. Under the same chromatographic operating conditions, the sample is dissolved in chloroform, and tetradecane is used as an internal standard. figure 1 It is the gas chromatogram of oxadiazone standard sample, wherein the...
Embodiment 2
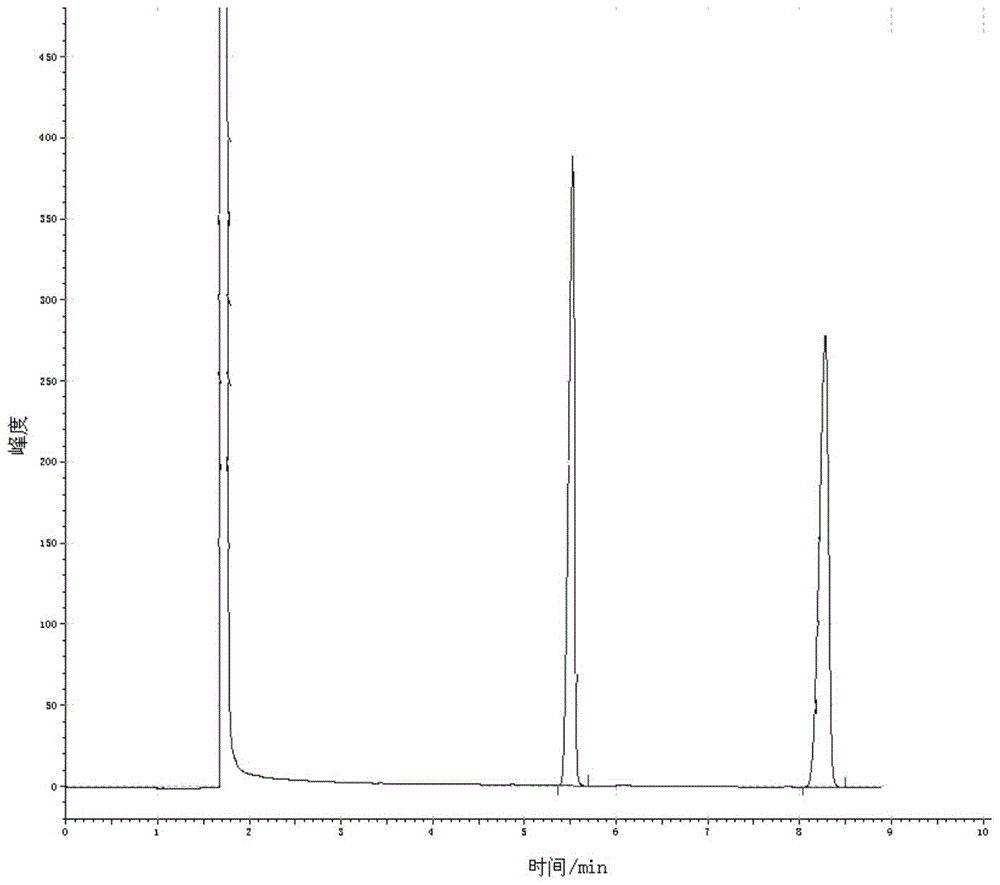
[0046] Add 5.0Kmol of 2,4-dichloro-5-isopropoxyphenylhydrazide and an appropriate amount of dichloroethane solution into the reaction kettle, stir and dissolve, and slowly add 6.0Kmol of methyl chloroformate solution dropwise in 2 hours, After 1.5 hours of dropwise addition, keep warm for 1.5 hours, slowly raise the temperature to reflux, and reflux for 5 hours. Sampling and detection of hydrazide is 0, and the reaction is complete. Add catalyst anhydrous sodium acetate 0.2Kmol, and keep warm at 60-70°C for 4 hours. During the reaction, the The by-product methanol was released, and after the reaction was completed, the temperature was lowered, washed with water, crystallized, and dried to obtain 1695.4 kg of oxadiazone product. It is detected by gas chromatography. Under the same chromatographic operating conditions, the sample is dissolved in chloroform, and tetradecane is used as an internal standard. figure 1 It is the gas chromatogram of oxadiazone standard sample, wherein...
Embodiment 3
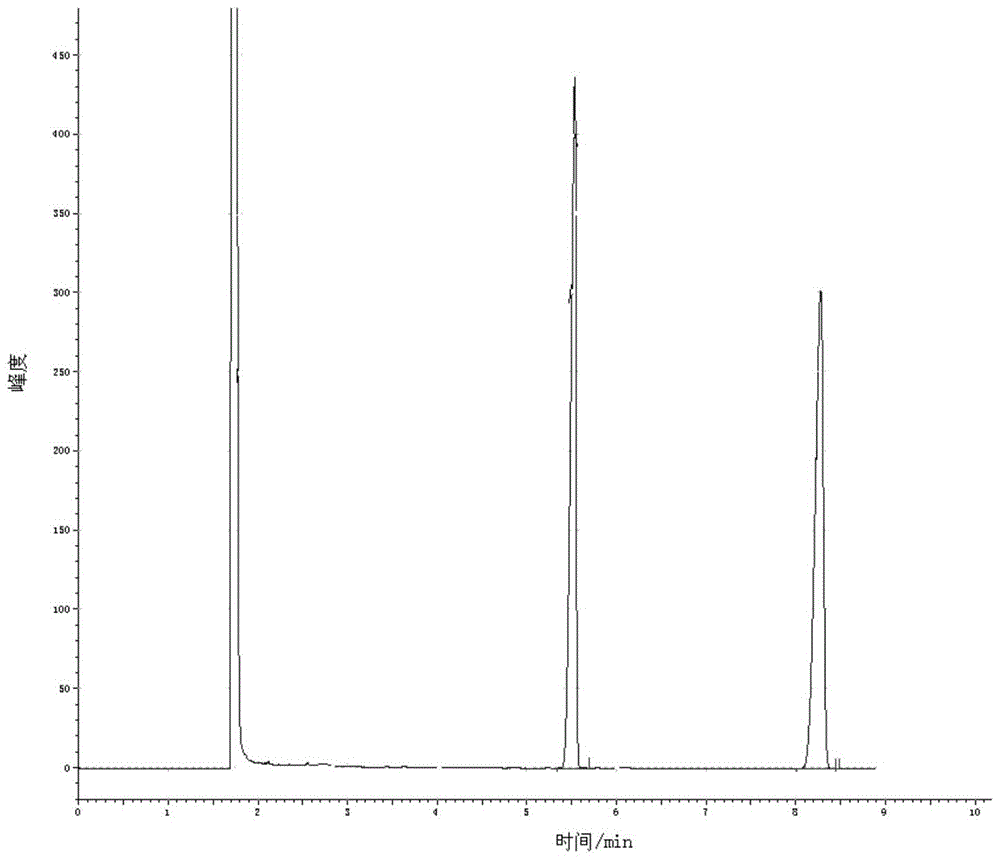
[0048] Add 2.0Kmol of 2,4-dichloro-5-isopropoxyphenylhydrazide and an appropriate amount of dichlorobenzene solution into the reaction kettle, stir to dissolve, slowly add the metered methyl chloroformate solution 3Kmol dropwise, and complete the dropwise addition within 1 hour , keep warm for 1h, slowly raise the temperature to reflux, reflux for 0.5h, take a sample to detect that the residual hydrazide is more than 0.5%, continue the reflux reaction for 1h, take a sample to detect that the residual hydrazide is 0.2%, regard it as the end of the reaction, add the crushed catalyst sodium hydroxide 0.1Kmol, heat up to 180-200°C and keep it warm for 3 hours. During the reaction, the by-product methanol is released. After the reaction, the temperature is lowered, washed with water, crystallized, and dried to obtain 685.3kg of oxadiazone product. It is detected by gas chromatography. Under the same chromatographic operating conditions, the sample is dissolved in chloroform, and tet...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com