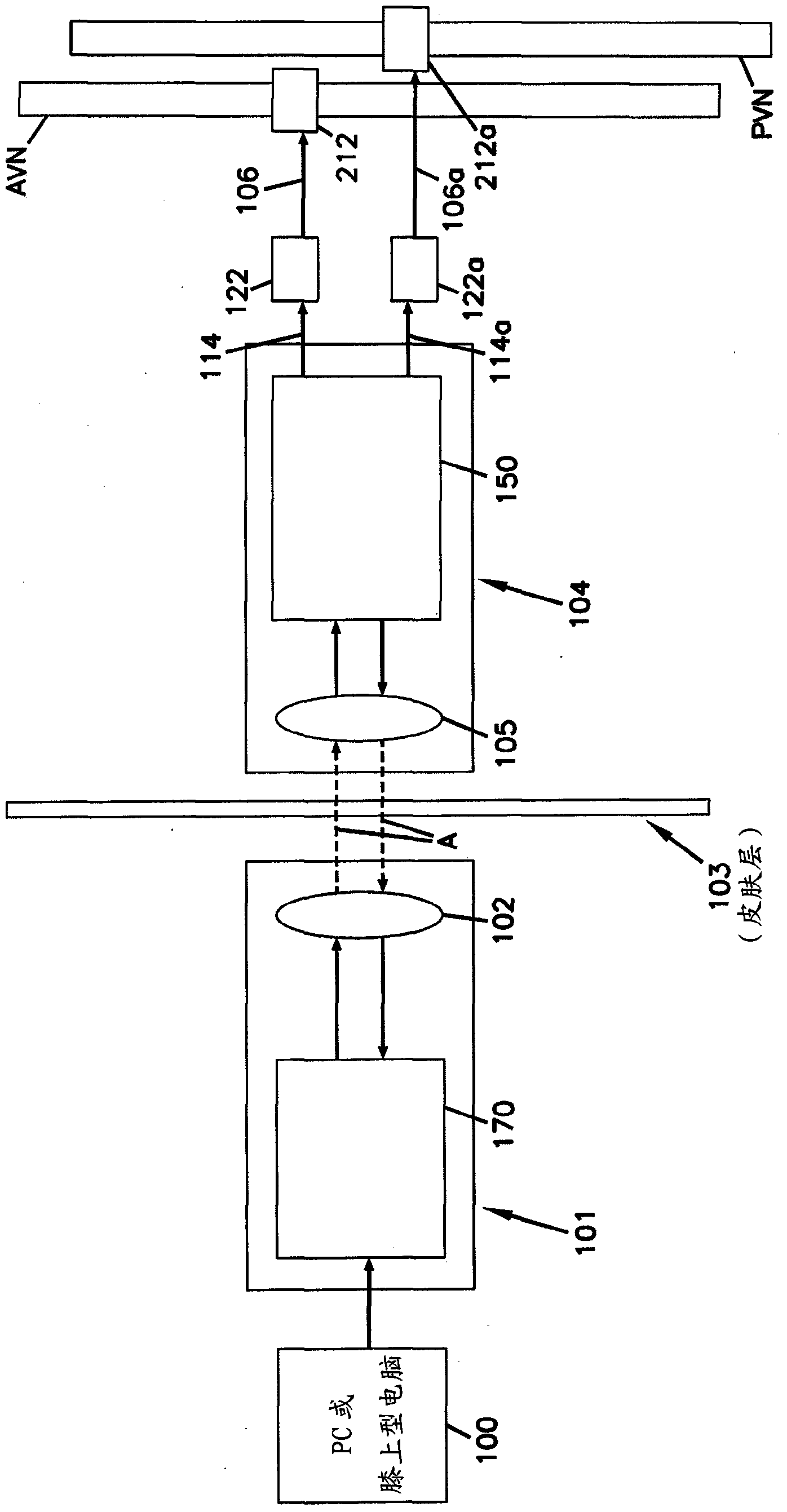
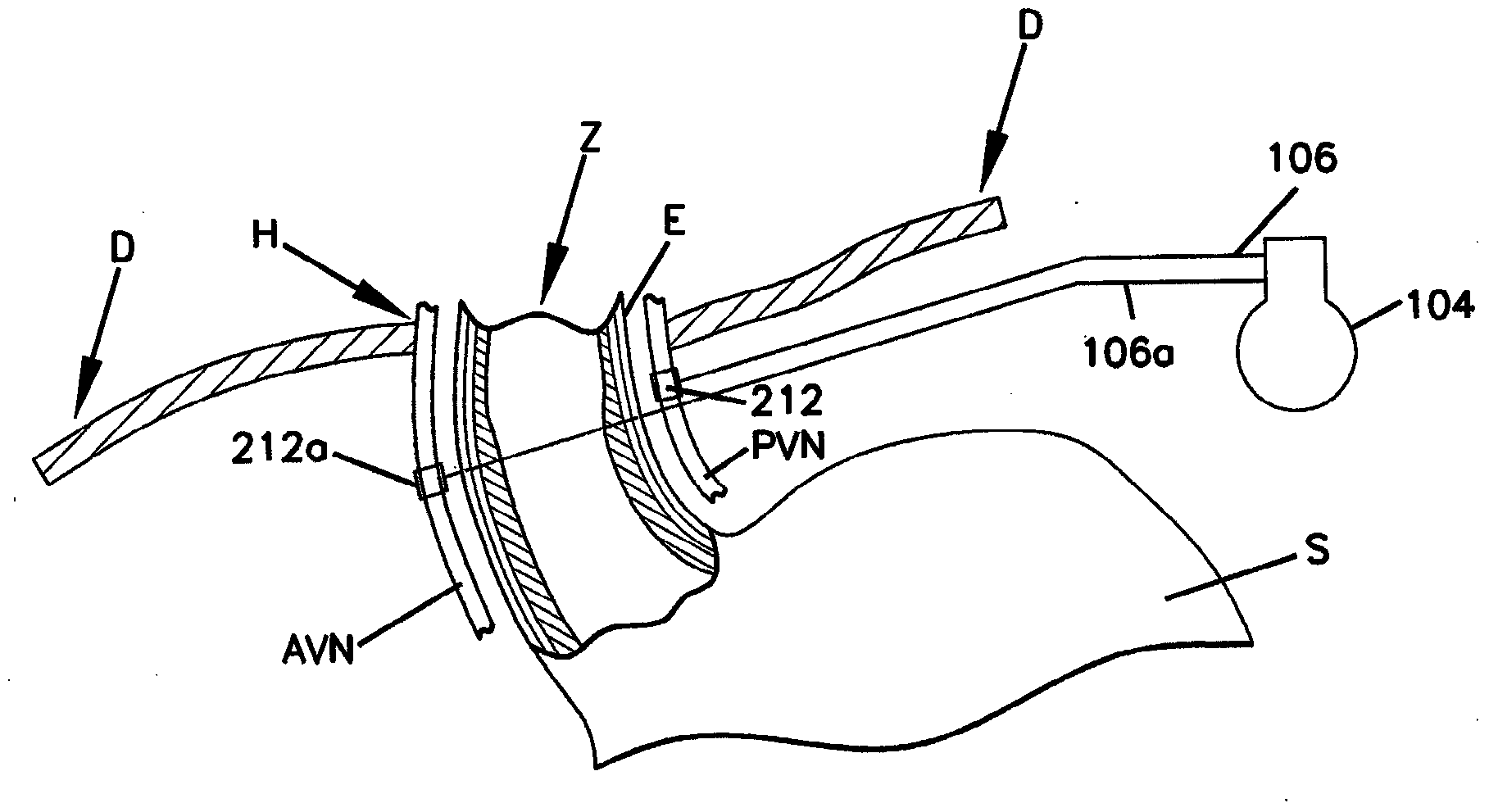
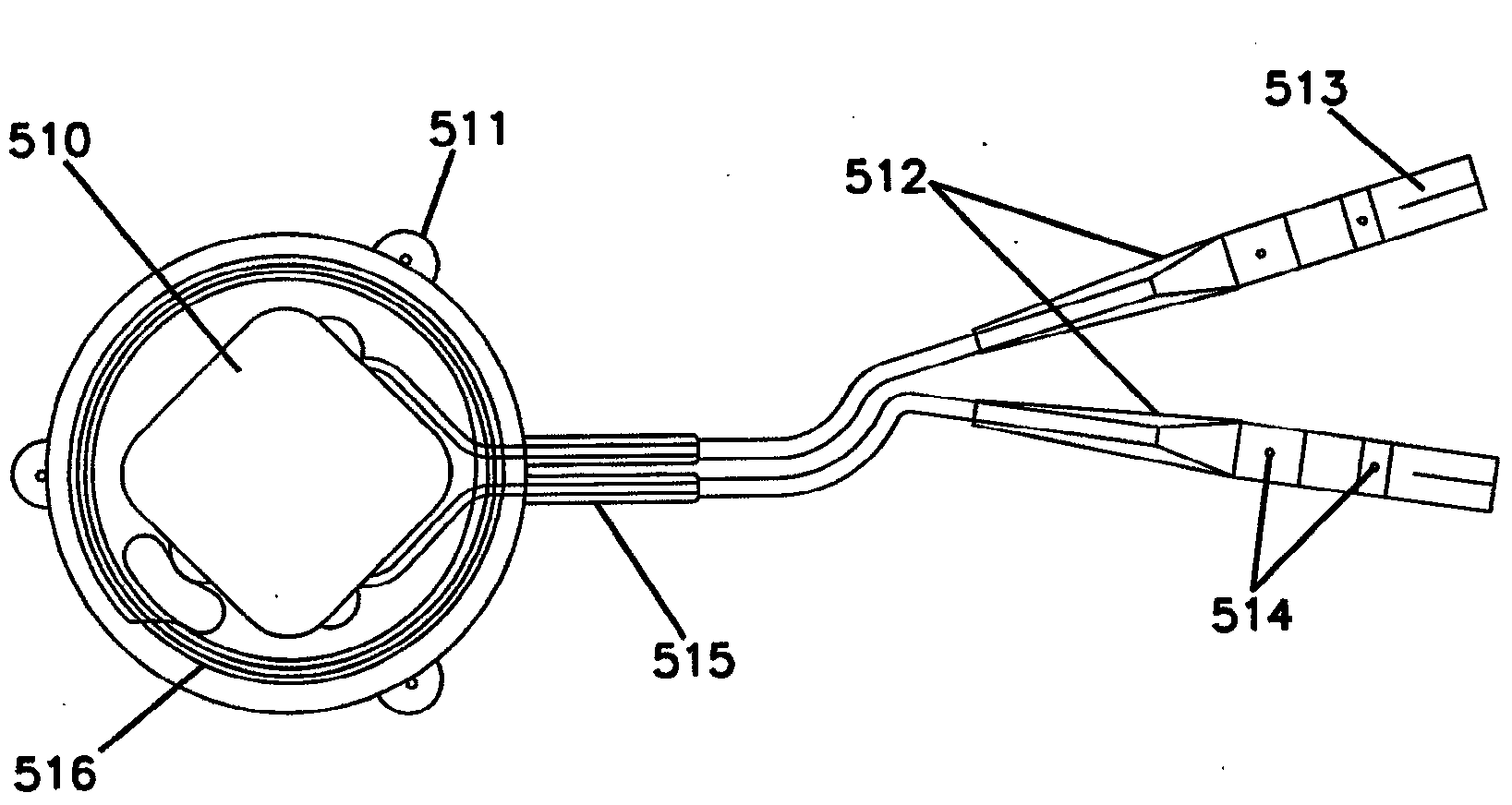
Devices for regulation of blood pressure and heart rate
A blood pressure and regulator technology, applied in applications, cardiovascular system diseases, treatment, etc., can solve problems such as insufficient control of blood pressure or heart rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0234] Materials and methods / experimental design
[0235] An open-label, prospective, baseline-controlled, four-center clinical study was conducted to evaluate the feasibility and safety and efficacy of a device as described herein that induces intermittent electrical blockade of the anterior and posterior vagal trunks . Participating centers include Flinders Medical Centre, Adelaide, Australia; Circle of Care, Sydney, Australia; University Hospital, Basel , Switzerland; and St. Olavs University Hospital, Trondheim, Norway.
[0236] patient
[0237] Male or female obese subjects (BMI31.5-55kg / m) aged 25-60 years (inclusive) were recruited at four centers 2 ). The study evaluated device safety and efficacy for up to 6 months.
[0238] The ability to complete all study visits and procedures is an eligibility requirement. Relevant exclusion criteria included: current type 1 diabetes mellitus (DM) or type 2 DM poorly controlled with oral hypoglycemic agents or with associate...
Embodiment 2
[0295] Materials and methods
[0296] Research design
[0297] This study is a prospective, open-label, multicenter study evaluating the safety and efficacy of a high-frequency electrical algorithm applied to the intra-abdominal vagal trunk to promote weight loss and improve glycemic control in type 2 diabetes control and blood pressure. Subjects' pre-implantation baseline measurements were used as controls.
[0298] The study was carried out at the following sites: National Institute of Nutrition (Instituto National de la Nutricion, INNSZ), Mexico City, Mexico; Trondheim University Hospital, Trondheim, Norway; University Hospital, Basel, Switzerland; Flinders Medical Centre, Adelaide, Australia; and Institute of Weight Control , Sydney (Sydney), Australia. This study is registered with clinicaltrials.gov (NCT00555958).
[0299] research subjects
[0300] Obese female and male subjects with type 2 diabetes (body mass index (BMI) 30-40kg / m 2 (endpoints inclusive), age 25...
Embodiment 3
[0332] This study was a multicenter, prospective, randomized, double-blind, controlled, parallel-group trial with a 12-month post-randomization follow-up period. All subjects in both groups received Maestro at implantation (EnteroMedics Inc, St. Paul, MN) for all implantable components. Obese subjects without diabetes at initial therapy were randomized in a 2:1 allocation to treatment and control groups. A limited number of patients with type 2 diabetes were randomized with a 1:1 allocation. At the end of the blinded 1-year follow-up period, all subjects received open-label VBLOC therapy and continued to be followed for an additional 4 years.
[0333] Research center
[0334] Fifteen academic and / or private practice clinics participated in the EMPOWER study (see list of contributing centers). All surgeons involved in the VBLOC feasibility study or have received Maestro placement in the classroom and in the animal laboratory under the supervision of a laparoscopic surgeon ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com