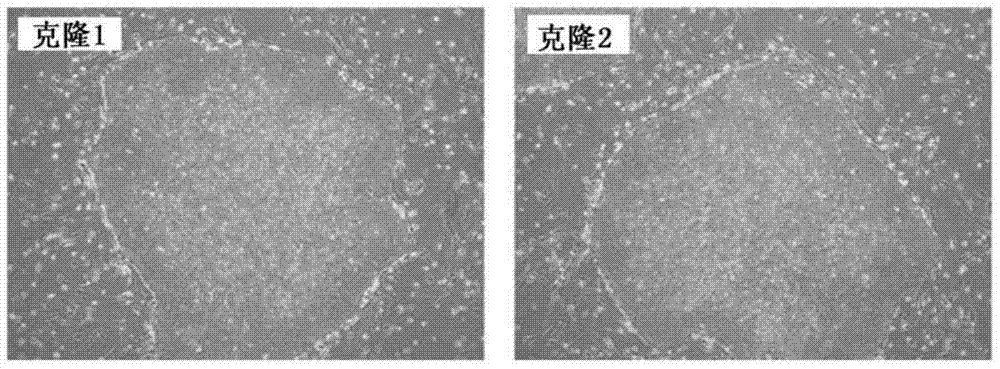
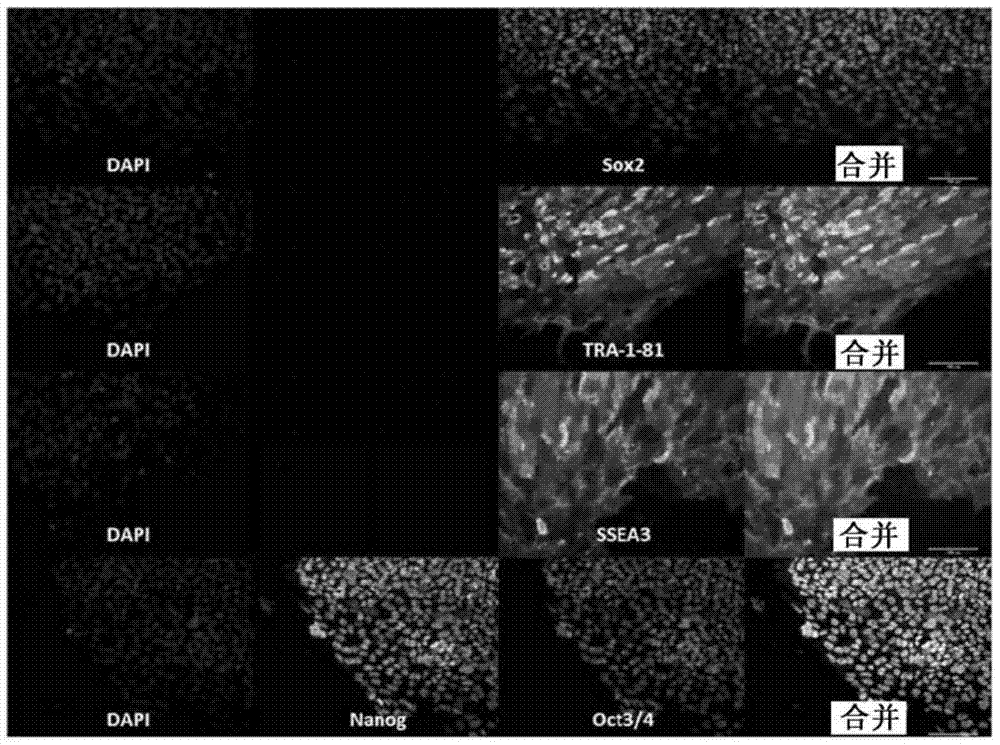
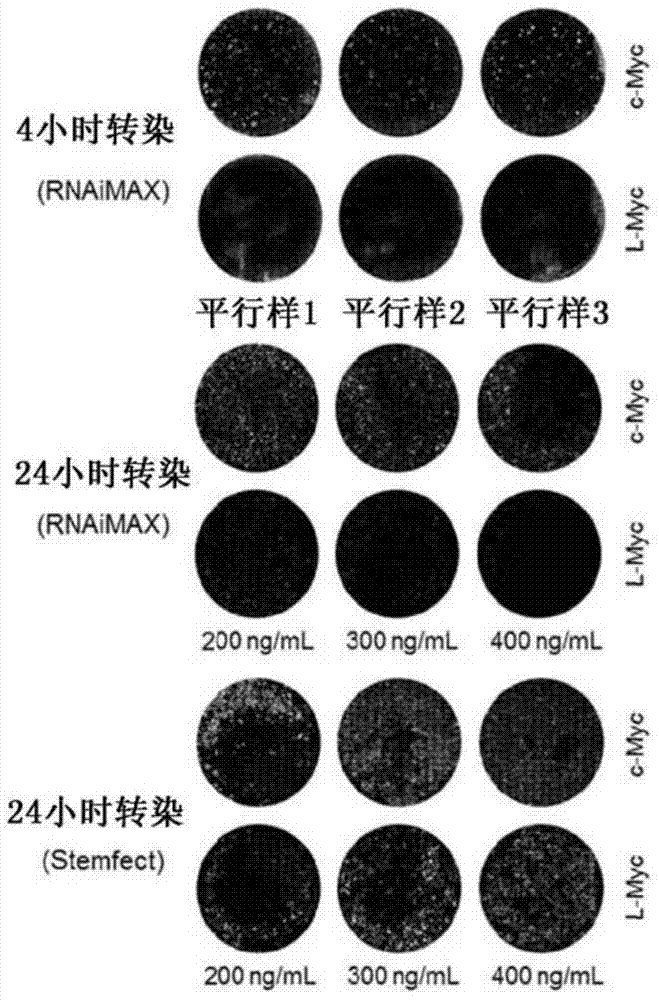
Feeder-free derivation of human-induced pluripotent stem cells with synthetic messenger RNA
A cell and somatic cell technology, applied in the direction of genetically modified cells, cell culture active agents, artificially induced pluripotent cells, etc., can solve the problem of low efficiency of pluripotent cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-I
[0075] The generation of embodiment 1-IVT template
[0076] Plasmid constructs for generating templates for in vitro transcription (IVT) of linear PCR products were constructed using ligation-independent cloning (LIC). We first constructed parental plasmids (pIVT) incorporating 5' and 3' untranslated regions (UTRs) flanked by open reading frame (ORF) inserts designed to accept encoding proteins of interest the insertion site. The sequences flanking the ORF were as described in Warren et al., Cell Stem Cell, 2010, comprising a low secondary structure leader sequence and a strong Kozak site (5'UTR) and murine α-globin 3'UTR. A linearized version of the pIVT vector with a 5' overhang was generated by re-annealing of two PCR products amplified from the plasmid using tailed primers. ORF PCR products with complementary overhangs were generated by a similar procedure, combined with vector PCR products, and transformed into DH5α bacteria by heat shock to clone gene-specific constr...
Embodiment 2
[0077] Example 2 – Generation of mRNA Mixtures
[0078] The mRNA synthesis process is outlined in Figure 5. Synthetic mRNA was generated using a 4:1 ratio of ARCA cap analog to GTP in the IVT reaction to generate a high percentage of capped transcripts. Complete replacement of CTP by 5m-CTP and UTP by pseudo-UTP in a nucleotide triphosphate (NTP) mixture was used to reduce the immunogenicity of the RNA product. Cap analogs and modified NTPs were purchased from Trilink Biotechnologies. Prepare a 2.5x NTP mix (ARCA:ATP:5m-CTP:GTP:pseudo-UTP of 15:15:3.75:3.75:3.75 mM) to replace the standard NTP supplied with the MEGAscript T7 kit (Ambion) used to perform the IVT reaction . Each 40 µL IVT reaction contained 16 µL of NTP mix, 4 µL of 10x T7 buffer, 16 µL of DNA template, and 4 µL of T7 enzyme. Reactions were incubated at 37°C for 4-6 hours and then treated with 2 μL of TURBO DNase for an additional 15 minutes at 37°C before purification on MEGAclear (Ambion) spin columns el...
Embodiment 3
[0079] Example 3 - Cells and media
[0080] Reprogrammed target cells include BJ neonatal fibroblasts (ATCC), HDF-f fetal fibroblasts, HDF-n neonatal fibroblasts and HDF-a adult fibroblasts (ScienCell) and XFF xenobiotic-free neonatal Fibroblasts (Millipore). For BJ, HDF and XFF cells, expansion cultures were in BJ Medium (DMEM+10% FBS), ScienCell Fibroblast Medium and FibroGRO Xeno-free Human Fibroblast Expansion Medium (Millipore) conduct. The feeder cells used were 3001G irradiated neonatal human foreskin fibroblasts (GlobalStem) and FibroGRO mitomycin C-inactivated xeno-free human neonatal fibroblasts (Millipore). Cell passaging steps associated with xeno-free feeder-based and feeder-free reprogramming assays were performed using TrypLE Select (Gibco), an animal product-free cell dissociation reagent.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap