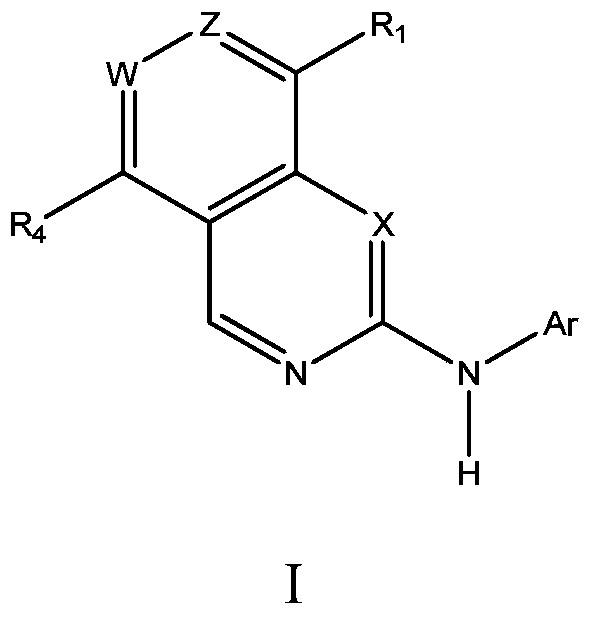
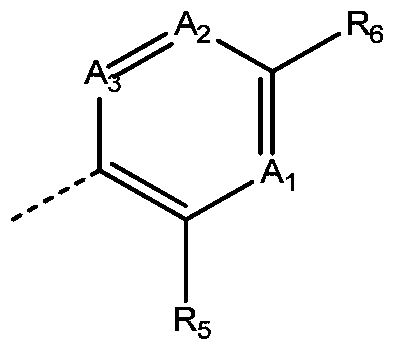
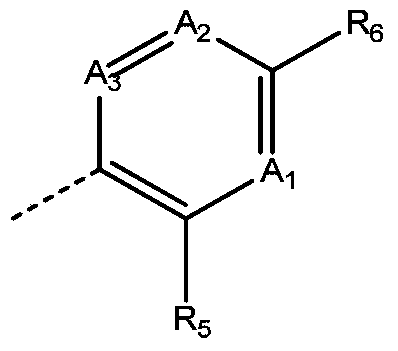
inhibitor compound
A technology of compound, alkyl, applied in the field of compound of spindle checkpoint function
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0881] (4-((5-(1-(2-(dimethylamino)ethyl)-1H-pyrazol-4-yl)isoquinolin-3-yl)amino)-3-methoxyphenyl )(3-methoxyazetidin-1-yl)methanone
[0882]
[0883] method 1
[0884] Make 2-(4-(3-chloroisoquinolin-5-yl)-1H-pyrazol-1-yl)-N,N-dimethylethylamine (preparation 1, 10mg, 0.033mmol), (4 -Amino-3-methoxyphenyl)(3-methoxyazetidin-1-yl)methanone (Formulation 28, 15.7 mg, 0.066 mmol), xantphos (11.5 mg, 0.02 mmol), Pd 2 (dba) 3 (3mg, 0.003mmol) and Cs 2 CO 3 (87 mg, 0.27 mmol) in toluene / DMF (3 / 1 mL) was stirred at 160 °C for 2 hours under microwave irradiation. The reaction mixture was filtered, diluted with NaCl solution and extracted with EtOAc. The organic layer was washed with 2M NH 3 Purified on an SCX-2 column eluting with / MeOH and concentrated in vacuo. The residue was purified by Biotage silica gel column chromatography eluting with 0-4% MeOH in EtOAc, followed by preparative HPLC to afford the title compound (5 mg, 30%).
[0885] 1 H NMR (500MHz, CDCl 3 ):δ9.07...
Embodiment 19
[0902] (3-methoxy-4-((5-(1-(1-methylpiperidin-4-yl)-1H-pyrazol-4-yl)isoquinolin-3-yl)amino)phenyl )(3-methoxyazetidin-1-yl)methanone
[0903]
[0904] To (3-methoxy-4-((5-(1-(piperidin-4-yl)-1H-pyrazol-4-yl)isoquinolin-3-yl)amino)phenyl)(3 A solution of -methoxyazetidin-1-yl)methanone (Example 20, 18 mg, 0.035 mmol) in DCM / MeOH (4 / 2 mL) was added with acetic acid (2.5 μL, 0.044 mmol) and aqueous formaldehyde solution (38% w / w, 6.0 μL, 0.073 mmol), followed by sodium triacetoxyborohydride (11.2 mg, 0.053 mmol). The reaction mixture was stirred at room temperature for 1 hour. The solvent was removed and the residue was washed with 2M NH 3 Purification by SCX-2 column eluting with / MeOH afforded the title compound (17 mg, 92%) as a yellow oil.
[0905] 1 H NMR (500MHz, CDCl 3 ): δ9.05(d, J=0.9Hz, 1H), 8.01(d, J=8.3Hz, 1H), 7.82(dt, J=8.3, 1.1Hz, 1H), 7.78(d, J=0.8Hz ,1H),7.69(d,J=0.8Hz,1H),7.54(dd,J=7.1,1.2Hz,1H),7.49(t,J=1.0Hz,1H),7.40-7.32(m,3H) ,7.20(dd,J=8.4,1.9Hz,...
Embodiment 20
[0909] (3-methoxy-4-((5-(1-(piperidin-4-yl)-1H-pyrazol-4-yl)isoquinolin-3-yl)amino)phenyl)(3- Methoxyazetidin-1-yl)methanone
[0910]
[0911] 4-(4-(3-((2-methoxy-4-(3-methoxyazetidine-1-carbonyl)phenyl)-amino)isoquinoline-5 -yl)-1H-pyrazol-1-yl)piperidine-1-carboxylic acid tert-butyl ester (Example 3, 23 mg, 0.038 mmol) in DCM (8 mL) was added TFA (0.5 mL). The reaction was stirred at room temperature for 16 hours. The solvent was removed in vacuo and the residue was washed with 2M NH 3 Purification by SCX-2 column eluting with / MeOH afforded the title compound (19 mg, 98%) as a yellow oil.
[0912] 1 H NMR (500MHz, CDCl 3 ):δ9.05(d,J=0.9Hz,1H),8.00(d,J=8.3Hz,1H),7.81(d,J=8.1Hz,1H),7.77(s,1H),7.69(s ,1H),7.54(dd,J=7.1,1.3Hz,1H),7.50(s,1H),7.40-7.30(m,3H),7.20(dd,J=8.3,1.9Hz,1H),4.55- 4.35(m,2H),4.35-4.30(m,1H),4.29-4.20(m,2H),4.15-4.05(m,1H),3.96(s,3H),3.33(s,3H),3.32- 3.27 (m, 2H), 2.87-2.78 (m, 2H), 2.30-2.22 (m, 2H), 2.04-1.92 (m, 2H).
[0913] LCMS (ESI) Rt = ...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap