Novel crystal form of Tipracil hydrochloride and preparation method thereof
A technology of crystal form and hydrochloric acid, applied in the field of new crystal form of Tipracil hydrochloride and its preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
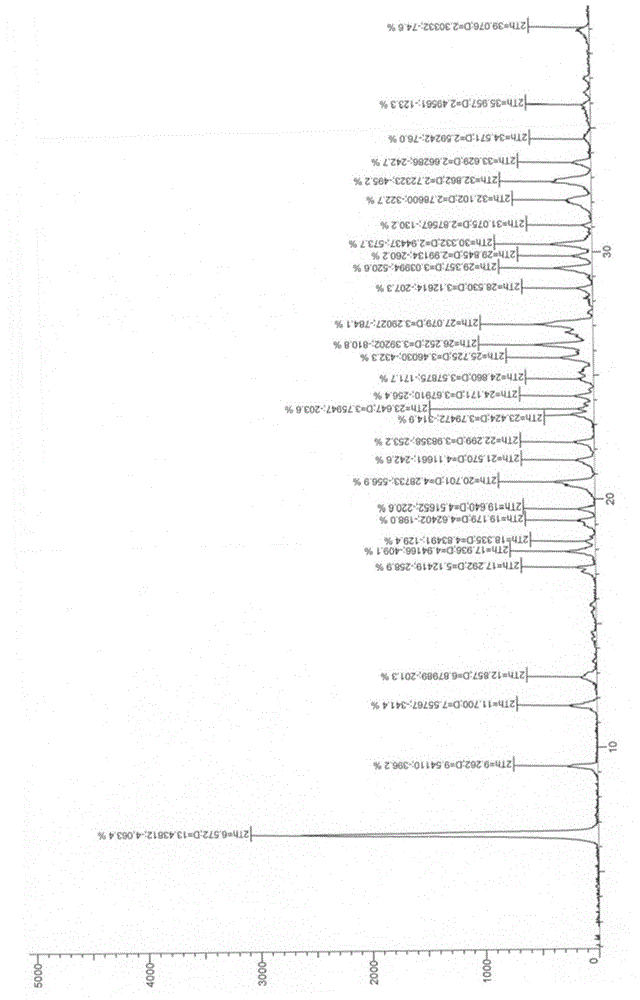
Image
Examples
Embodiment 1
[0027] Embodiment 1 uses dilute hydrochloric acid to prepare the crystal form II of Tipiracil hydrochloride
[0028] Add compound 1 (50g, 0.18mol) into purified water (500ml), stir at 20°C until dissolved, then add 50ml of concentrated hydrochloric acid dropwise, during the dropwise addition, crystals gradually precipitate out, and after dropping, continue stirring at this temperature Crystal 2h. After filtering, the resulting solid was air-dried at 40°C until constant weight. The target product (41.5 g, white solid) was obtained with a yield of 83%.
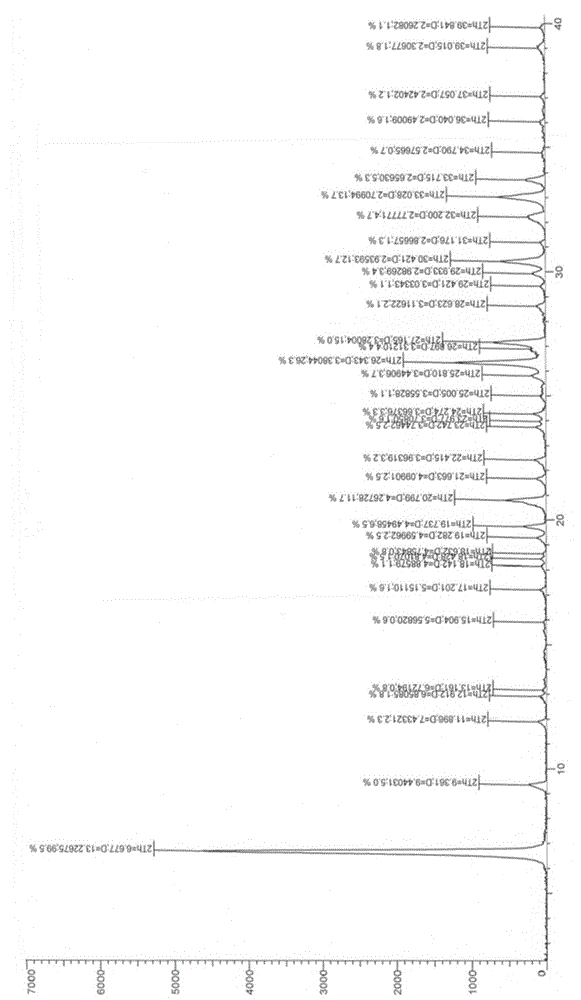
[0029] figure 2 is the X-ray diffraction pattern of the crystal form II of the sample prepared in Example 1.
Embodiment 2
[0030] Embodiment 2 uses acetone hydrochloride to prepare the crystal form II of Tipiracil hydrochloride
[0031] Compound 1 (50 g, 0.18 mol) was added into purified water (500 ml), stirred at 20° C. until dissolved, and a mixed solvent of 25 ml of hydrochloric acid and 2 L of acetone was added to the clear liquid. After continuing to stir at this temperature for about 20 minutes, crystals gradually precipitated out, and continued to stir and crystallize for 2 hours. After filtering, the resulting solid was air-dried at 40°C until constant weight. The target product (43.5 g, white solid) was obtained with a yield of 87%.
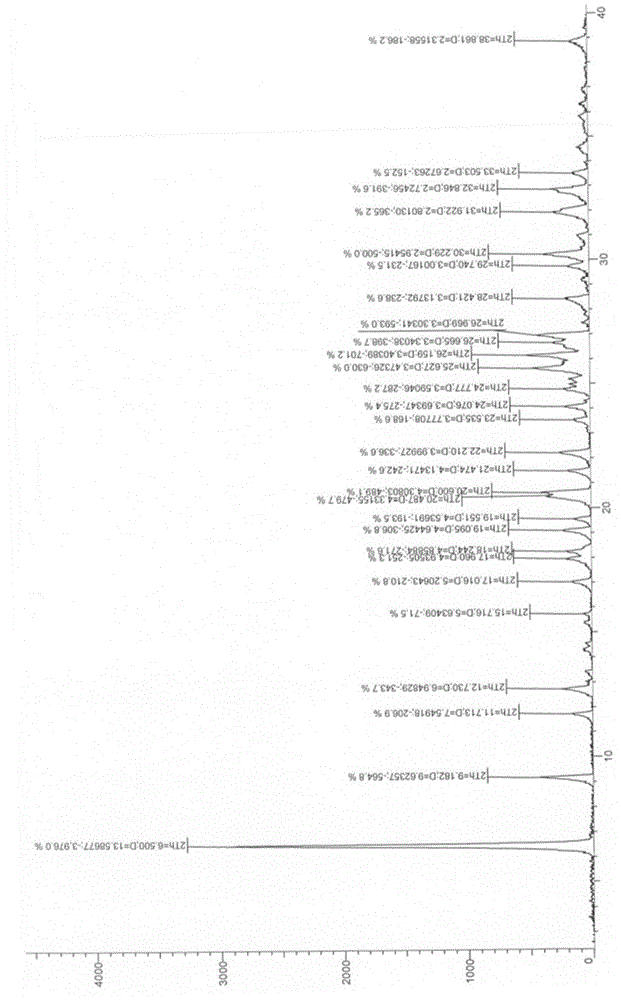
[0032] image 3 is the X-ray diffraction pattern of the crystal form II of the sample prepared in Example 2.
PUM
| Property | Measurement | Unit |
|---|---|---|
| strength | aaaaa | aaaaa |
| strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com