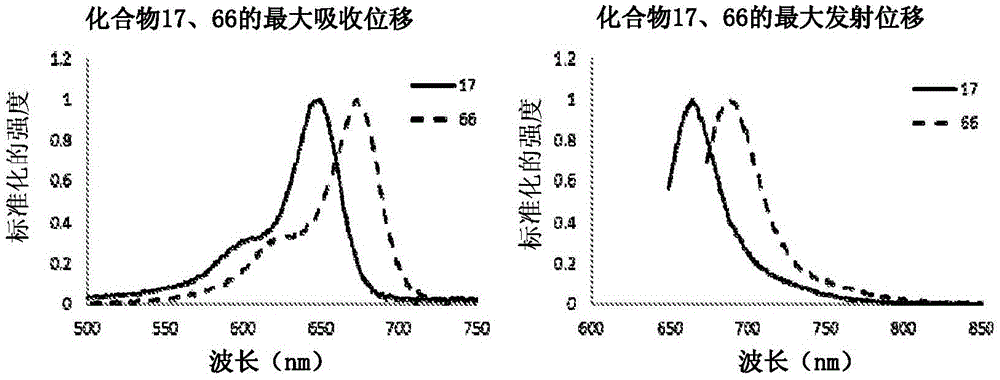
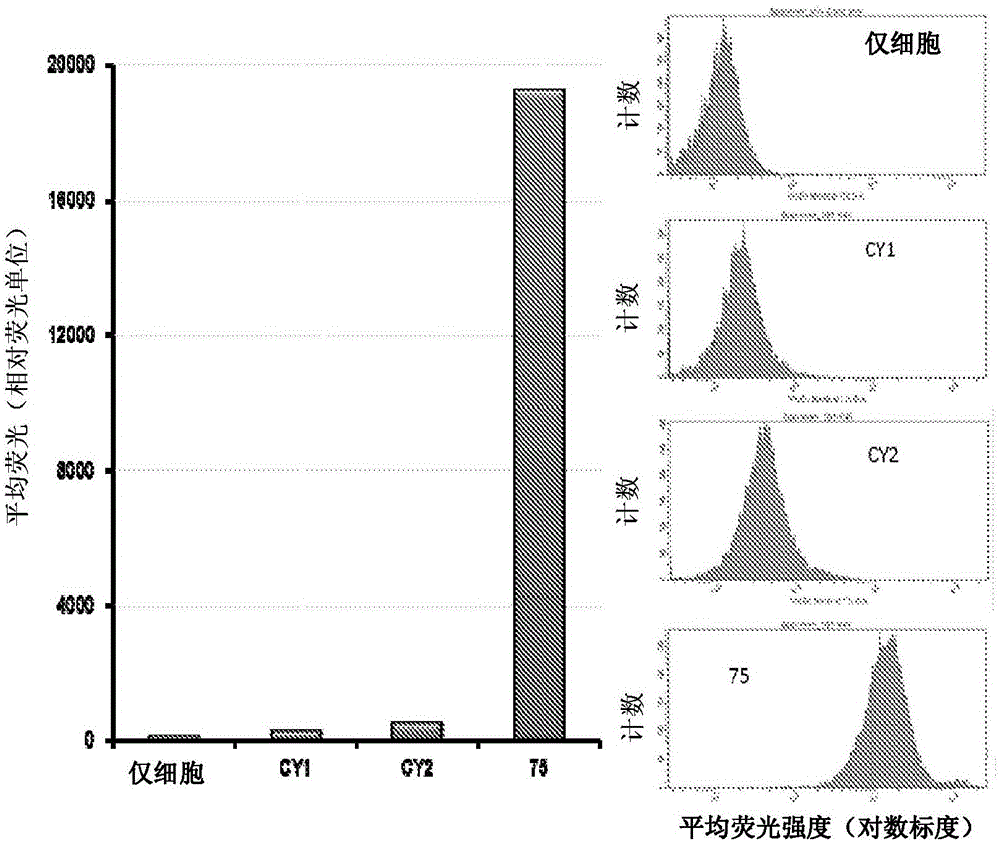
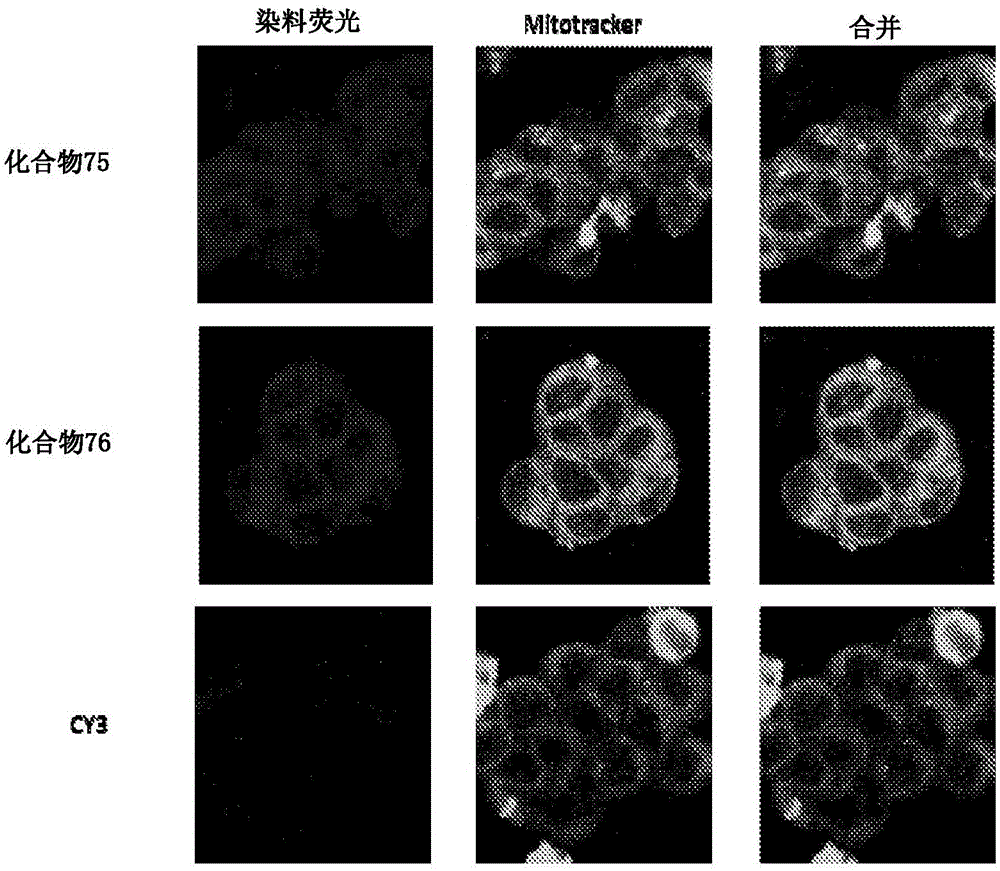
Substituted silaxanthenium red to near-infrared fluorochromes for in vitro and in vivo imaging and detection
一种取代基、杂环基的技术,应用在体内试验用的配制品、二苯氧杂芑胺/氧杂蒽酮/噻吨酮/硒代呫吨酮/碲杂呫吨酮染料、有机染料等方向,能够解决体积大、限制应用、低细胞膜通透性等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0355] Example 1-Compound 22 (3,6-bis(dimethylamino)-9-(2-carboxy-4-methyl-thiophen-5-yl)-10,10-dimethyl-10-sila Anthracene cation chloride) synthesis
[0356] Compound 22 was synthesized according to the following scheme:
[0357]
[0358] To a solution of compound N,N-dimethyl-3-bromoaniline (10.0 g, 50.0 mmol) in AcOH (250 mL) was added 12.16 mL of 37% aqueous formaldehyde (4.5 g, 150.0 mmol), and the mixture was stirred at 60° C. for 115 minute. After cooling to room temperature, a portion of the acetic acid was removed by vacuum. Then, with saturated NaHCO 3 (aq.) and NaOH (aq.) to neutralize the reaction mixture, and CH 2 Cl 2 extract. The organic layer was washed with concentrated saline solution (brine), in Na 2 SO 4and evaporated to dryness. The residue was purified by flash chromatography (silica gel) to yield pure 4,4'-methylenebis(3-bromo-N,N-dimethylaniline) (5.24 g, 12.7 mmol, 51% yield).
[0359]
[0360] To a nitrogen-purged flask was added 4,4'...
Embodiment 2
[0363] Example 2-Compound 65 (3,6-bis(dimethylamino)-9-(2-carboxy-thiophen-5-yl)-10,10-dimethyl-10-siloxanthene cation chloride) Synthesis
[0364]
[0365] In a nitrogen-purged flask, 3,6-bis(dimethylamino)-10,10-dimethyl-10-siloxanthone (50.0 mg, 0.16 mmol) was dissolved in anhydrous THF (10 mL) . The solution was cooled to -78°C. At the same temperature, tert-butyl 4-bromo-3-methyl-2-thiophenecarboxylic acid (136 mg, 0.81 mmol) and anhydrous THF (5 mL) were added to the flask, and 1M sec-butyllithium (1.16 mL, 1.62 mmol), and the mixture was stirred for 30 minutes. The lithiation solution was added slowly, and the mixture was warmed to room temperature, then stirred for 2 hours. The reaction was blocked by the addition of 2N HCl, and the mixture was stirred at room temperature for 10 minutes. Add saturated NaHCO 3 , and with CH 2 Cl 2 Extract it all. The organic layer was in Na 2 SO 4 Dry under , and evaporate the solvent. The crude mixture was purified by HP...
Embodiment 3
[0366] Example 3-Compound 18 (3,6-bis(dimethylamino)-9-(2-carboxy-3-methyl-thiophen-4-yl)-10,10-dimethyl-10-sila Anthracene cation chloride) synthesis
[0367]
[0368] In a nitrogen-purged flask, 3,6-bis(dimethylamino)-10,10-dimethyl-10-siloxanthone (50.0 mg, 0.16 mmol) was dissolved in anhydrous THF (10 mL) . The solution was cooled to -78°C. At the same temperature, 3-methyl-4-bromo-2-thiophenecarboxylic acid (136 mg, 0.81 mmol) and anhydrous THF (5 mL) were added to the flask, and 1M sec-butyllithium (1.16 mL, 1.62 mmol) was added , and the mixture was stirred for 30 minutes. The lithiation solution was added slowly, and the mixture was warmed to room temperature, then stirred for 2 hours. The reaction was blocked by the addition of 2N HCl, and the mixture was stirred at room temperature for 10 minutes. Add saturated NaHCO 3 , and with CH 2 Cl 2 Extract it all. The organic layer was in Na 2 SO 4 Dry under , and evaporate the solvent. The crude mixture was pu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com