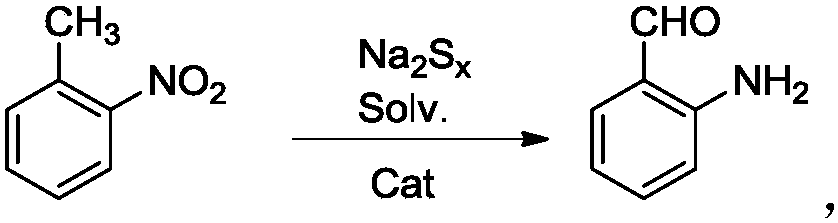A kind of efficient, fast method for synthesizing o-aminobenzaldehyde
A technology of o-aminobenzaldehyde and o-nitrotoluene, which is applied in chemical instruments and methods, preparation of organic compounds, organic compound/hydride/coordination complex catalysts, etc., can solve slow progress, long reaction time, yield, etc. Rate difference and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
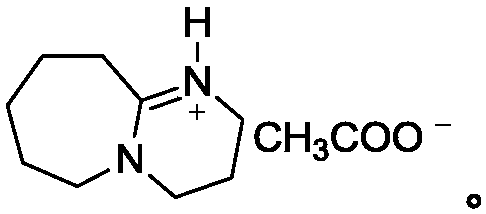
[0024] Put sodium polysulfide solution (0.1mol) in a 100mL three-neck flask, add 50mL of anhydrous methanol and 5mmol ionic liquid under stirring, heat to 50°C, slowly add o-aminotolualdehyde (0.1mol) dropwise, and then reflux and stir After 2 hours, HPLC followed detection. After the raw materials disappeared, the reaction solution was extracted with dichloromethane, the organic phases were combined, the organic solvent was removed under reduced pressure, and the product was obtained after vacuum drying. The yield was 87.1%, and the content was 96.5% (HPLC detection).
Embodiment 2
[0026] Put sodium polysulfide solution (0.1mol) in a 100mL three-neck flask, add 50mL of anhydrous methanol and 5mmol ionic liquid under stirring, heat to 50°C, slowly add o-aminotolualdehyde (0.1mol) dropwise, and then reflux and stir After 3 hours, HPLC traced and detected that the raw materials disappeared. The reaction solution was extracted with dichloromethane, the organic phases were combined, the organic solvent was removed under reduced pressure, and the product was obtained after vacuum drying with a yield of 90.2% and a content of 96.8% (detected by HPLC).
Embodiment 3
[0028] Put sodium polysulfide solution (0.1mol) in a 100mL three-neck flask, add 50mL of anhydrous methanol and 5mmol ionic liquid under stirring, heat to 50°C, slowly add o-aminotolualdehyde (0.1mol) dropwise, and then reflux and stir After 5 hours, HPLC tracking detection showed that the raw materials disappeared. The reaction solution was extracted with dichloromethane, the organic phases were combined, the organic solvent was removed under reduced pressure, and the product was obtained after vacuum drying with a yield of 92.6% and a content of 96.2% (HPLC detection).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com