Medicinal composition for treating asthma and application thereof
A composition and drug technology, applied in the field of pharmaceutical compositions for the treatment of asthma, can solve the problems of untimely treatment, large adverse reactions, arrhythmia, etc., and achieve the effect of lasting effect, high safety, and improving lung function
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] The preparation of embodiment 1 Chinese medicine dry ointment powder
[0015] Weigh juvenile red 1.2kg, sword bean 1.8kg, acacia seed 0.7kg, basil 0.8kg, terrestris flower 0.8kg, alder bark 1.2kg, acacia bark 1.0kg, Baiwei 1.0kg, magnolia 0.8kg, holy basil 0.8kg and Yulinghua 0.8kg, put it in a sandwich pot and mix evenly, add water to cover the medicinal surface 2cm, soak for 30 minutes, heat to boil, then simmer for 1 hour, filter after cooling, add water 10 times the total mass of the medicinal materials to the filter residue After heating and boiling, decoct with low heat for 1 hour, then filter, combine the two filtrates, concentrate to obtain extract, dry at 80°C to obtain dry extract, and then crush the dry extract into fine powder of 120 mesh to obtain dry extract powder of traditional Chinese medicine extract.
Embodiment 2
[0016] The preparation of embodiment 2 Chinese medicine dry ointment powder
[0017] Weigh juvenile red 1.5kg, sword bean 1.9kg, acacia seed 0.8kg, basil 0.9kg, terrestris flower 0.9kg, alder bark 1.5kg, acacia bark 0.9kg, Baiwei 0.9kg, magnolia 0.7kg, holy basil 0.7kg Put 0.7kg of jade bell flowers in a sandwich pot and mix evenly, add water to cover the medicine surface 2cm, soak for 30 minutes, heat to boil, then simmer for 1h, cool and filter, add appropriate amount of water to the filter residue, heat to boil, then simmer for 1h , and then filtered, the two filtrates were combined, concentrated into an extract, dried at 80°C to obtain a dry extract, and then the dry extract was crushed into a fine powder of 120 mesh to obtain a dry extract powder of the Chinese medicine extract.
Embodiment 3
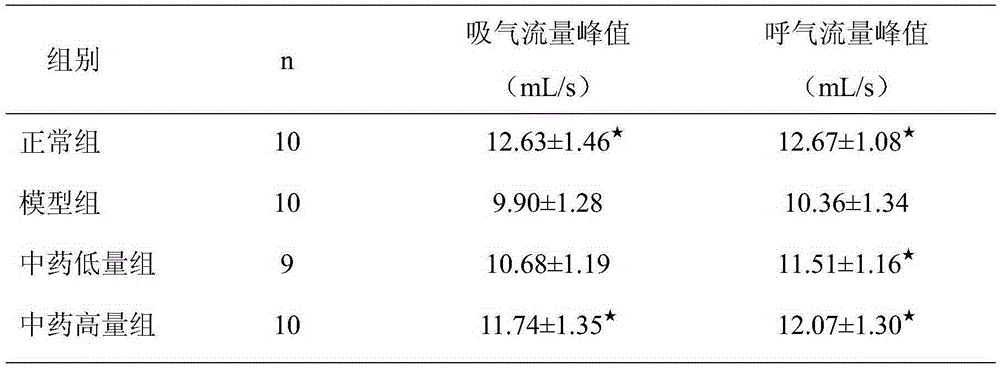
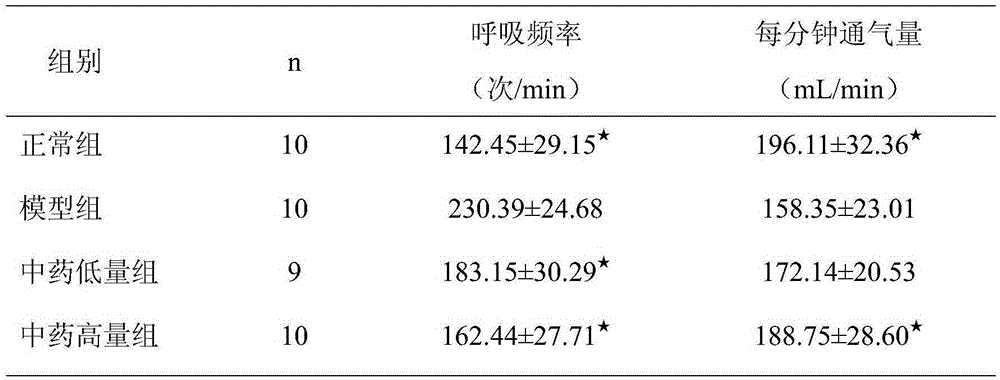
[0018] The curative effect research of embodiment 3 Chinese medicine extracts to asthmatic rats
[0019] 40 clean-grade SD rats, with a body weight ranging from 180 to 220 g, were fed adaptively for one week, and all rats were randomly divided into normal group, model group, and low-dose and high-dose Chinese medicine extract groups, with 10 rats in each group, male and female Half and half. Except for the normal group, rats in other groups were sensitized with ovalbumin (OVA) to prepare animal models of bronchial asthma. The method was as follows: 3 Dry powder 100mg normal saline suspension 1mL intraperitoneal injection for sensitization, repeat the sensitization once on the 8th day, start ultrasonic atomization 1% 0VA to stimulate from the 15th day, about 30min / time, at 8-9 am every day Proceed, and end the stimulation operation in the 4th week. During nebulization, the rats were found to have symptoms such as irritability, sneezing, incontinence, ear scratching, and cyano...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com