Method for stabilizing chlorpheniramine and salt thereof
A technology containing chlorpheniramine and chlorobenzene, which is applied in the fields of pharmaceutical formulations, allergic diseases, active ingredients of heterocyclic compounds, etc. It can solve the problems of maintaining chlorpheniramine and achieve the effect of inhibiting the reduction of content
Active Publication Date: 2016-01-27
SENJU PHARMA CO LTD
View PDF8 Cites 0 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
[0005] However, there are no reports on the adsorption properties of chlorpheniramine and/or its salts in neutral to alkaline liquid preparations to plastic containers, and the status quo is that, in the prior art, for chlorpheniramine con
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
 Login to View More
Login to View More PUM
 Login to View More
Login to View More Abstract
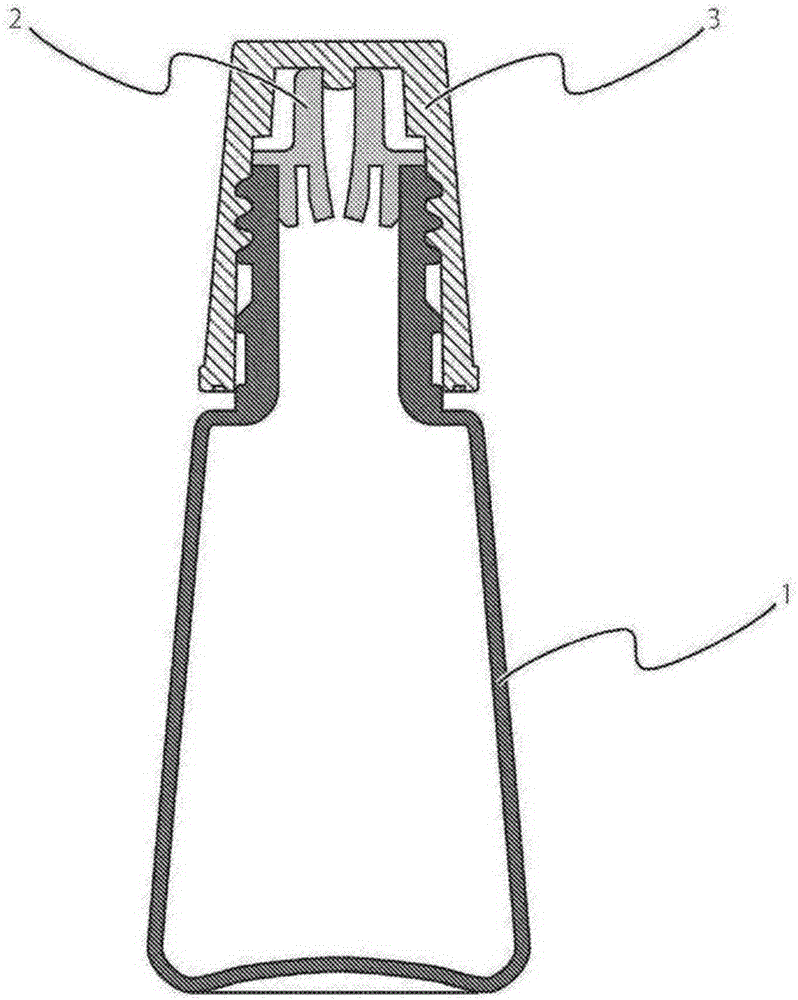
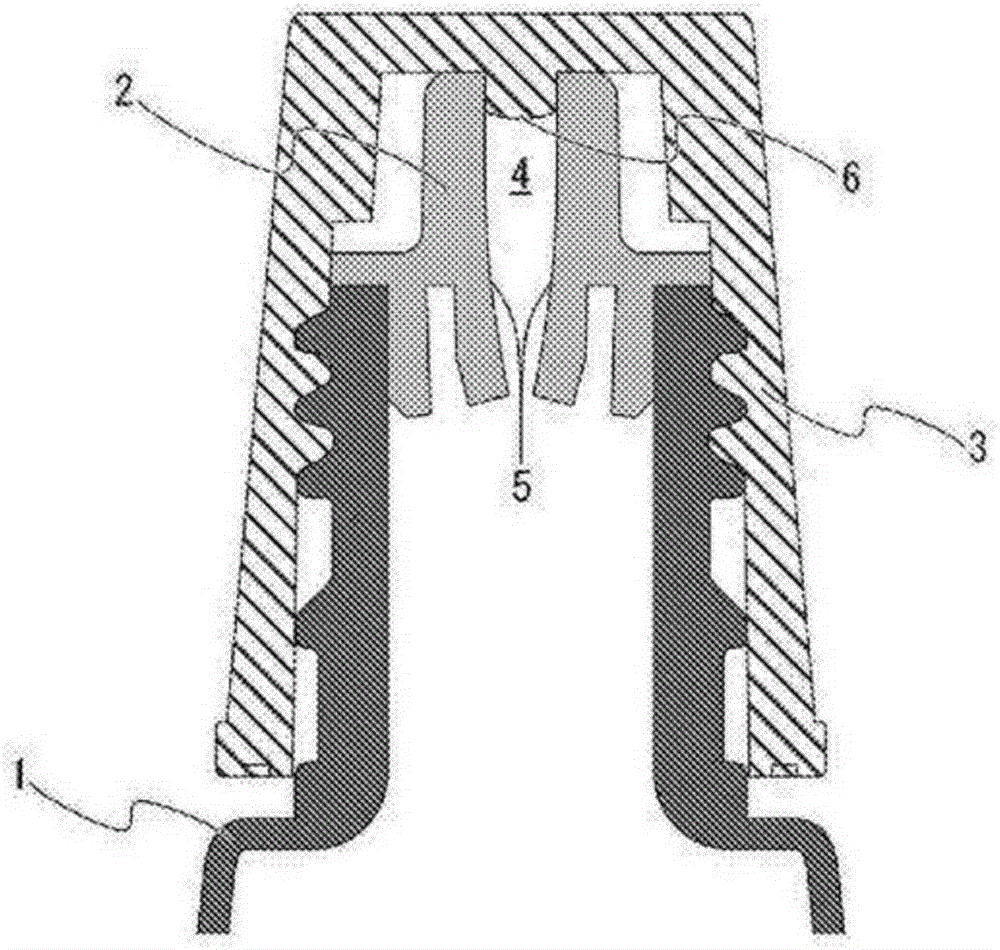
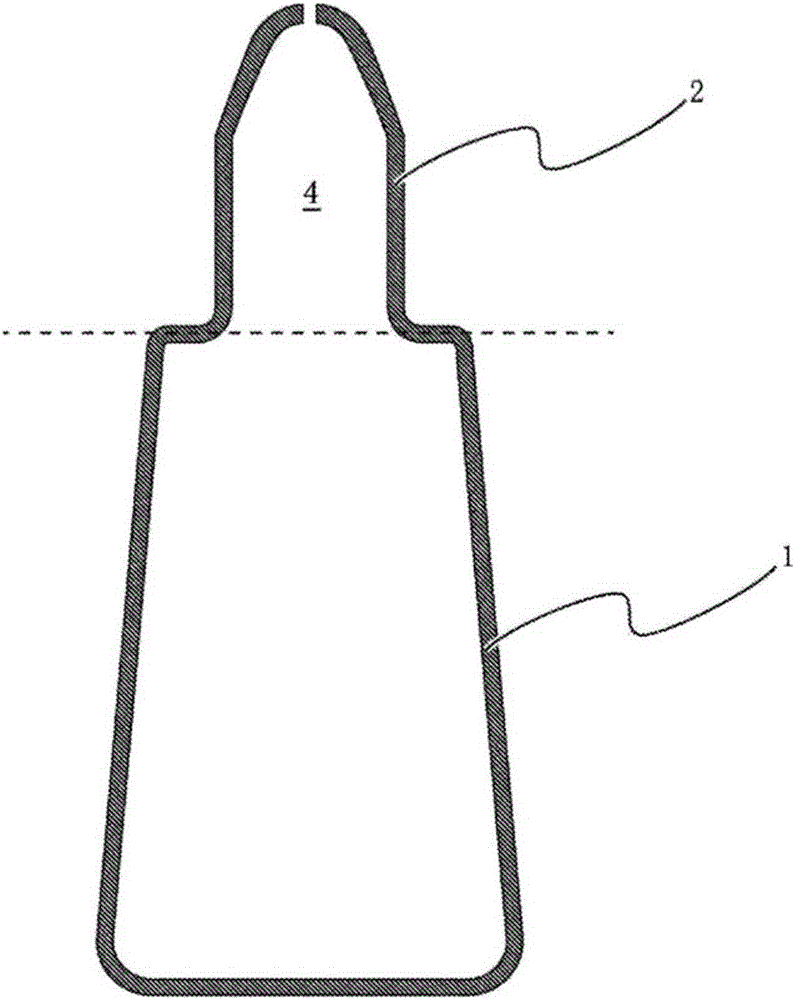
The purpose of the present invention is to provide a technique with which adsorption, onto a container, of chlorpheniramine and/or a salt thereof in a solution having a pH of at least 7.0 and including chlorpheniramine and/or the salt thereof, is inhibited, and the chlorpheniramine and/or the salt thereof is stably maintained. By employing a polybutylene terephthalate-containing resin as a resin for configuring inner wall surfaces (such as a wall surface around an internal space of a pour-out part and/or a lid wall surface facing a pour-out opening of the pour-out part) of a container for accommodating a solution which includes chlorpheniramine and/or a salt thereof, and which has a pH of at least 7.0, adsorption of the chlorpheniramine and/or the salt thereof onto the inner wall surfaces can be inhibited, and the content of the chlorpheniramine and/or the salt thereof in the solution can be stably maintained.
Description
technical field [0001] The invention relates to a product capable of stably maintaining a liquid formulation containing chlorpheniramine or a salt thereof and having a pH of 7.0 or higher. The present invention also relates to a method for stabilizing a liquid preparation containing chlorpheniramine or a salt thereof and having a pH of 7.0 or higher. Background technique [0002] Chlorpheniramine or a salt thereof is known to have an antihistamine action, and is used in pharmaceuticals such as eye drops, nasal drops, external skin preparations, and internal administration. At present, there have been many reports on formulation techniques utilizing chlorpheniramine or its salts. For example, it has been reported that a combination of chlorpheniramine or a salt thereof and pranoprofen or a salt thereof in a topical aqueous liquid preparation for ocular or nasal mucosa can impart excellent anti-inflammatory effects and antipruritic effects (Patent Document 1); By using Adapa...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): A61K31/4402A61J1/05A61K9/08A61P27/02
CPCA61K9/0048A61K31/4402A61P27/02A61P37/08A61K9/08A61K31/33B65D47/18
Inventor 根本夫规子
Owner SENJU PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com