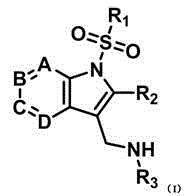
Indole and azaindole derivative and preparation method and application thereof in medicines
A compound and heteroaryl technology, applied in pharmaceutical formulations, antipyretics, antibacterials, etc., can solve the problems of slow onset of acid breakthrough at night, influence of administration time, instability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
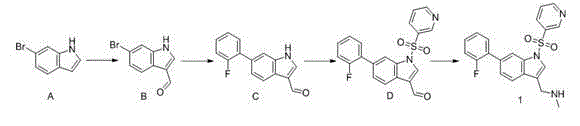
[0066] Embodiment 1: 1-(6-(2-fluoro-phenyl)-1-(pyridin-3-yl-sulfonyl)-1H-indol-3-yl)-N-methylmethylamine (compound 1 ) preparation
[0067]
[0068] 1) 6-Bromo-1 H - Preparation of indole-3-carbaldehyde (compound B)
[0069] At 0°C, POCl 3 (2.0mL) was added dropwise to DMF (8.0mL), stirred for 30 minutes, then added dropwise the mixture of compound 1 (3.0g, 15.3mmol) and DMF (2.0ml), after the addition was completed, naturally warmed to room temperature and stirred for 2 hours . After the reaction, the reaction solution was poured into ice water, and the pH was adjusted to about 8 with a saturated sodium hydroxide solution. Filtration, the filtrate was collected, and concentrated under reduced pressure to obtain a light yellow solid compound B (1.60g, 47%).
[0070] 1 H-NMR (400M, DMSO- d6 ) δ :12.24(bs,1H),9.94(s,1H),8.33(s,1H),8.03(d,1H),7.72(d,1H),7.36(d,1H)ppm.
[0071] 2) 6-(2-fluoro-phenyl)-1 H - Preparation of indole-3-carbaldehyde (compound C)
[...
Embodiment 2
[0080] Example 2: Preparation of N-methyl-1-(1-(pyridin-3-yl-sulfonyl)-1H-indol-3-yl)methanamine (compound 2)
[0081]
[0082] Referring to the preparation method of Compound 1, Compound 2 was obtained as a white solid with a yield of 40%. HPLC:93.1%; MS(ESI)m / z:[M+H] + =302.0; 1 H-NMR (400MHz, DMSO- d6 )δ:9.17(d,1H),9.09(br,1H),8.85(m,1H),8.45(m,1H),8.11(s,1H),7.99(d,1H),7.86(d,1H ), 7.64 (m, 1H), 7.42 (t, 1H), 7.37 (t, 1H), 4.27 (s, 2H), 2.53 (s, 3H) ppm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com