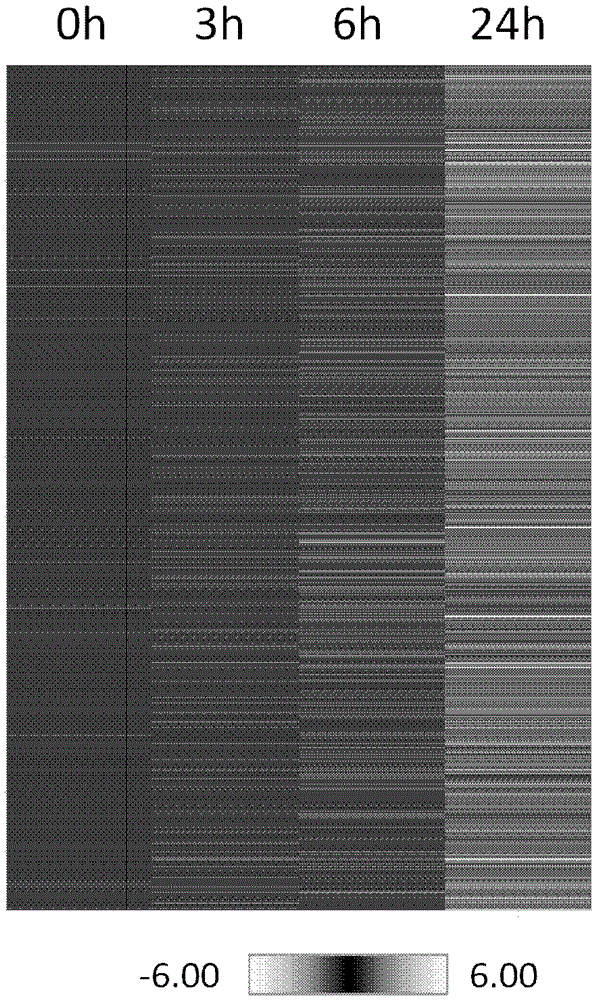
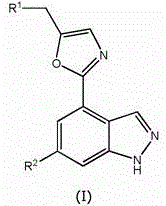
PI3K inhibitor for treatment of respiratory disease
A technology for the respiratory system and diseases, applied in the field of PI3K inhibitors for the treatment of respiratory diseases, which can solve problems such as inappropriate PI3Kδ activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
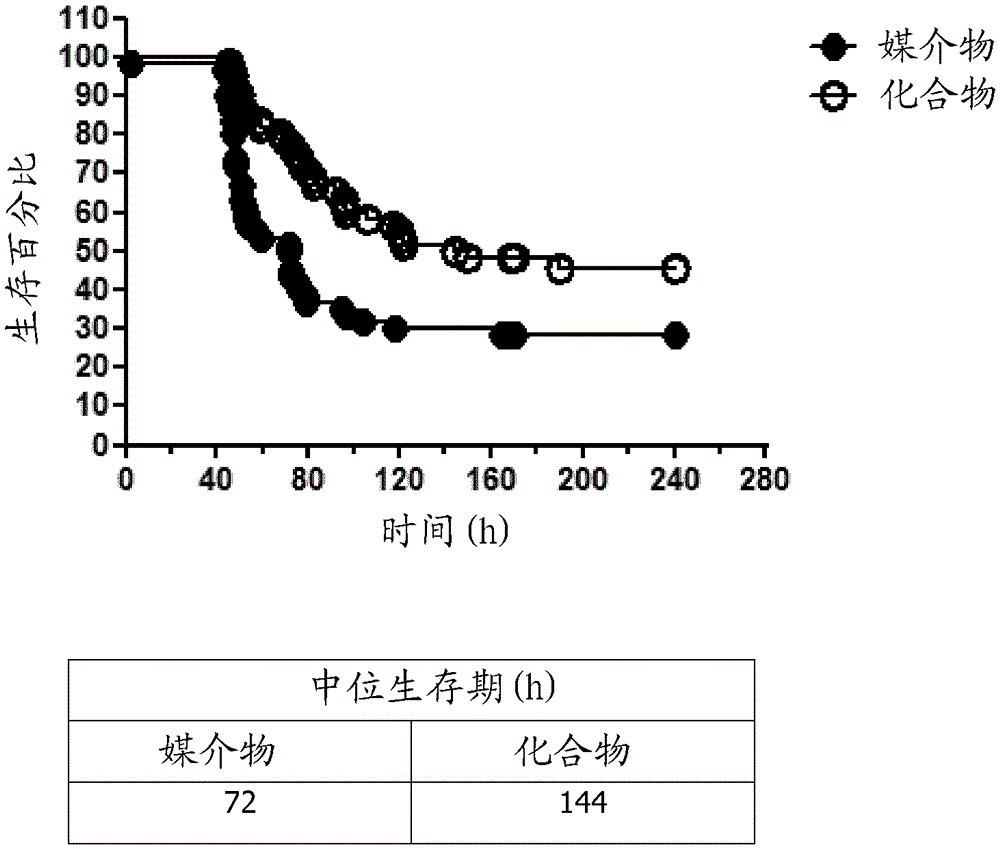
[0341] 6-(1H-indol-4-yl)-4-(5-{[4-(1-methylethyl)-1-piperazinyl]methyl}-1,3-oxazole-2- base)-1H-indazole hydrochloride for the treatment of Streptococcus pneumoniae
[0342] Germ-free C57BL / 6 male and female mice aged 10-12 weeks were intranasally administered 0.2% Tween-80 / saline vehicle or 0.2 mg / kg micronized 6-(1H-indole in the same vehicle Indol-4-yl)-4-(5-{[4-(1-methylethyl)-1-piperazinyl]methyl}-1,3-oxazol-2-yl)-1H-ind azole hydrochloride. Compound administration was performed twice daily for 11 days under anesthesia (induction with 3% isoflurane and maintenance with 2% isoflurane). Started on the second day and administered 6-(1H-indol-4-yl)-4-(5-{[4-(1-methylethyl)-1-piperazinyl]methyl}-1, One hour after 3-oxazol-2-yl)-1H-indazole hydrochloride or vehicle, mice were anesthetized with isoflurane as described above and treated with 1 × 10 7 CFU of intranasal infection with Streptococcus pneumoniae strain TIGR4. S. pneumoniae was obtained and prepared as previously ...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap