Multispecific antibodies, multispecific activatable antibodies and methods of using the same
A multi-specific and specific technology, applied in chemical instruments and methods, anti-receptor/cell surface antigen/cell surface determinant immunoglobulin, specific peptides, etc. The therapeutic use of clonal antibodies, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0530] Example 1: Preparation of multispecific antibodies
[0531] This example shows the construction, expression and purification of anti-Jagged-CD3, anti-Jagged-CTLA-4, anti-EGFR-CD3 and anti-EGFR-CTLA-4 multispecific antibodies.
[0532] The anti-Jagged (4D11v2) heavy chain, 5342-1204-4D11v2 light chain, anti-EGFRC225v5 heavy chain and 3954-1204-C225v5 light chain sequences shown above were expressed using vectors. Such vectors are described in co-pending applications PCT / US2013 / 038540, filed April 26, 2013 (title "Activatable Antibodies That Bind Epidermal Growth Factor Receptor And Methods Of Use Thereof") and PCT / US2013 / 047109, filed June 21, 2013 (title "Anti-Jagged Antibodies, Activatable Anti-Jagged Antibodies and Methods of Use Thereof"), the contents of each of which are incorporated herein by reference in their entirety.
[0533] The vector was digested with restriction enzymes NheI and NotI, and the vector fragments were separated by gel electrophoresis. Inse...
Embodiment 2
[0540] Example 2: Preparation of multispecific antibodies and multispecific activatable antibodies
[0541] Fully human IgG was expressed from transiently transfected HEK-293 cells. Co-transfection with the different heavy and light chain expression vectors shown in Table 10 enabled the expression of multispecific activatable antibodies.
[0542] Table 10
[0543] Transfection number
light chain carrier
heavy chain carrier
1
Anti-EGFR C225v5 LC
C225v5-OKT3 HC
97 --> 2
3954-1204-C225v5 LC
C225v5-OKT3 HC
3
C225v5 LC
C225v5-CTLA HC
4
3954-1204-C225v5 LC
C225v5-CTLA HC
5
Anti-Jagged 4D11v2 LC
4D11v2-OKT3 HC
6
5342-1204-4D11v2 LC
4D11v2-OKT3 HC
7
4D11v2 LC
4D11v2-CTLA HC
8
5342-1204-4D11v2 LC
4D11v2-CTLA HC
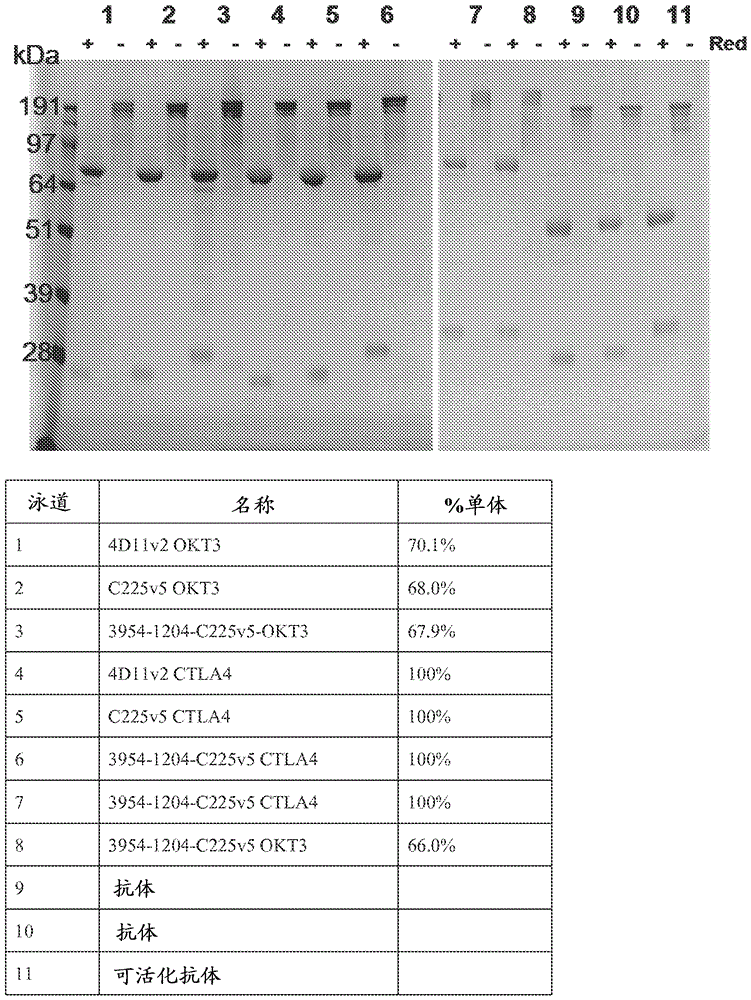
[0544]Multispecific antibodies and multispecific activatable antibodies expressed in HEK-293 cells were purified by protein A chrom...
Embodiment 3
[0548] Example 3: OKT3 binds to CD3ε
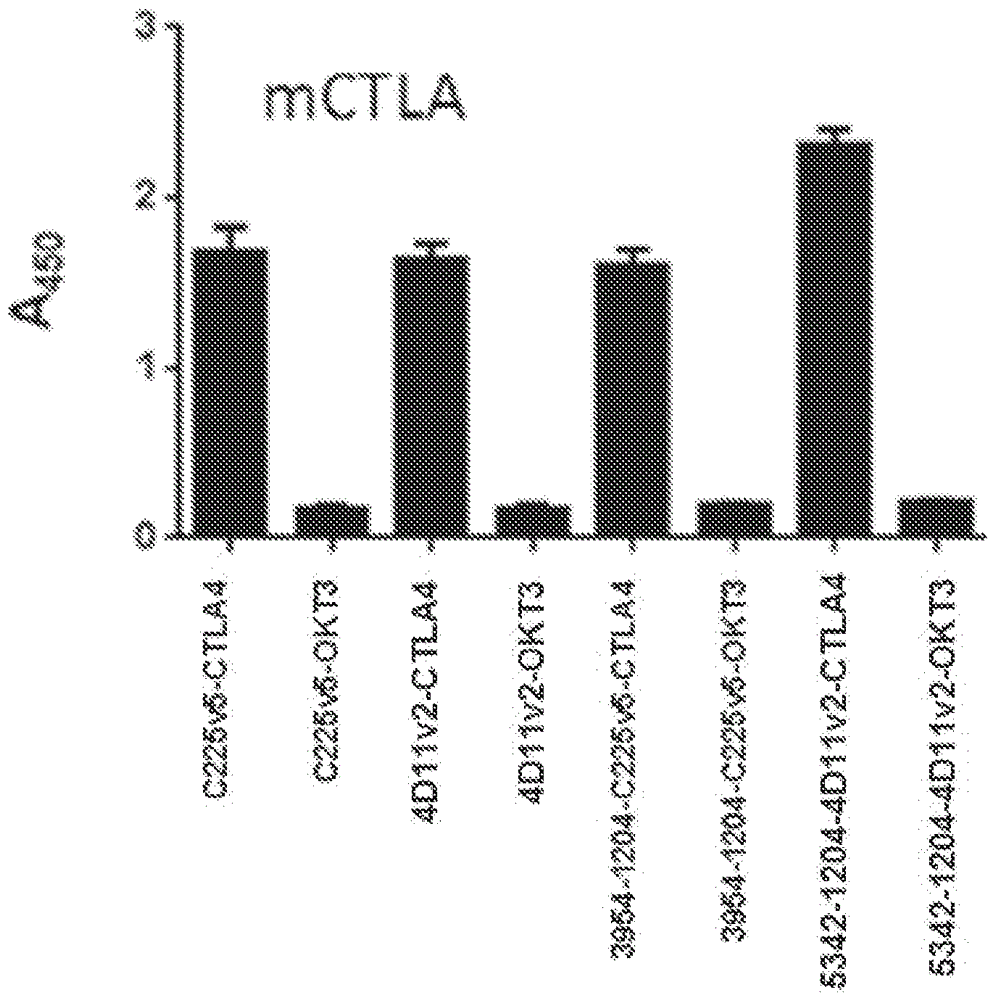
[0549] Such as Figure 13 As shown in , ELISA-binding experiments revealed that anti-EGFR multispecific activatable antibody 3954-1204-C225v5-OKT3 and anti-Jagged multispecific activatable antibody 5342-1204-4D11v2-OKT3 specifically bound human CD3ε. Human CD3ε (NovoProtein, Cat#C578) was adsorbed to the wells of a 96-well ELISA plate. The purified anti-EGFR multispecific activatable antibody 3954-1204-C225v5-CTLA-4, anti-EGFR multispecific activatable antibody 3954-1204-C225v5-OKT3, anti-Jagged multispecific activatable antibody 5342- 1204-4D11v2-CTLA-4 or anti-Jagged multispecific activatable antibody 5342-1204-4D11v2-OKT3 was applied to the plate and allowed to bind. Bound antibody was visualized with anti-human IgG-HRP conjugate (Fab-specific, Sigma, St Louis, MO; Cat#A0293-1ML) and developed with the chromogenic substrate TMB.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com