HLA-A11 restricted and carcino-embryonic antigen originated epitope peptide and application thereof
A HLA-A11, restricted technology, applied in the field of biomedicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
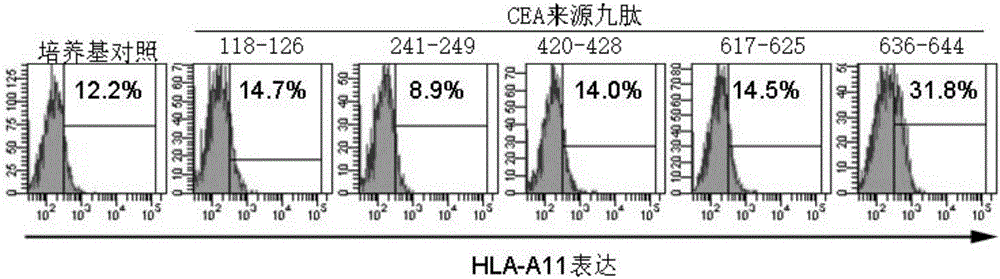
[0036] Example 1 Screening of HLA-A11 High Affinity Polypeptides
[0037] First, the primary sequence of the CEA antigen was analyzed by online biological software (SYFPEITHI, BIMAS and NetMHC) using HLA-A*1101, and 5 antigens that were likely to bind to HLA-A11 and induce the body to produce CTL were selectively synthesized peptide. See Table 1 for the sequence. We screened these peptides using LoVo cells because:
[0038] LoVo cells are colon tumor cells derived from HLA-A11 and highly expressing CEA. However, as detected by flow cytometry, LoVo expressed very low levels of MHC-I and HLA-A11. It is well known in the field of immunology that all nucleated cells in the body express MHC-I molecules, which can be regarded as antigen-presenting cells in a broad sense. The low expression of MHC-I in tumor cells, on the one hand, cannot effectively induce the body to produce CTL, on the other hand, it escapes being recognized by CTL and is cleared. One mechanism for the low ex...
Embodiment 2-4
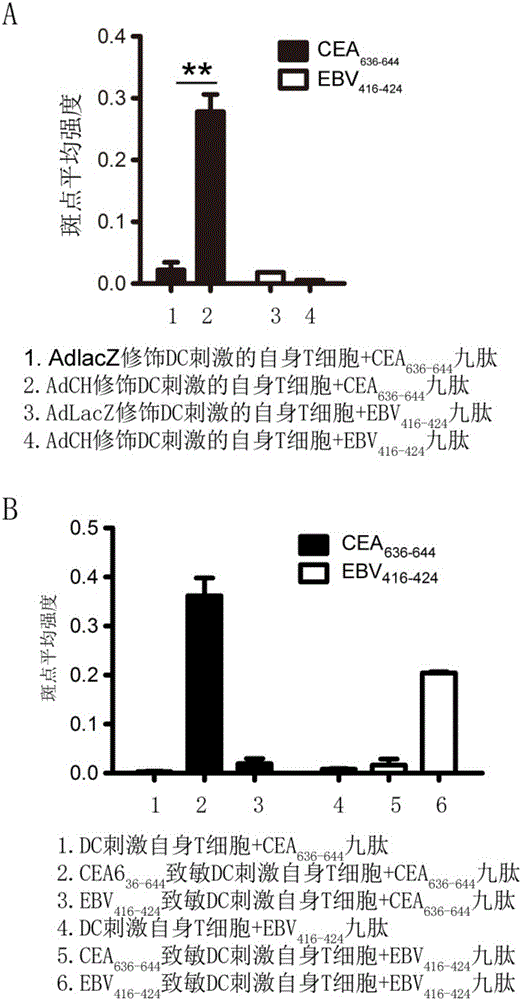
[0043] Example 2-4 Induction of HLA-A11-restricted CEA 636-644 Generation of specific CTL
[0044] 1. Preparation of human monocyte-derived dendritic cells (MoDC)
[0045] Separation of CD14 from peripheral blood of HLA-A11 positive healthy people by immunomagnetic bead method + Monocytes were immature MoDC after being induced by human recombinant GM-CSF (50ng / ml) and IL-4 (10ng / ml) for 5 days.
[0046] 2. Sensitization of DC
[0047] There are two methods:
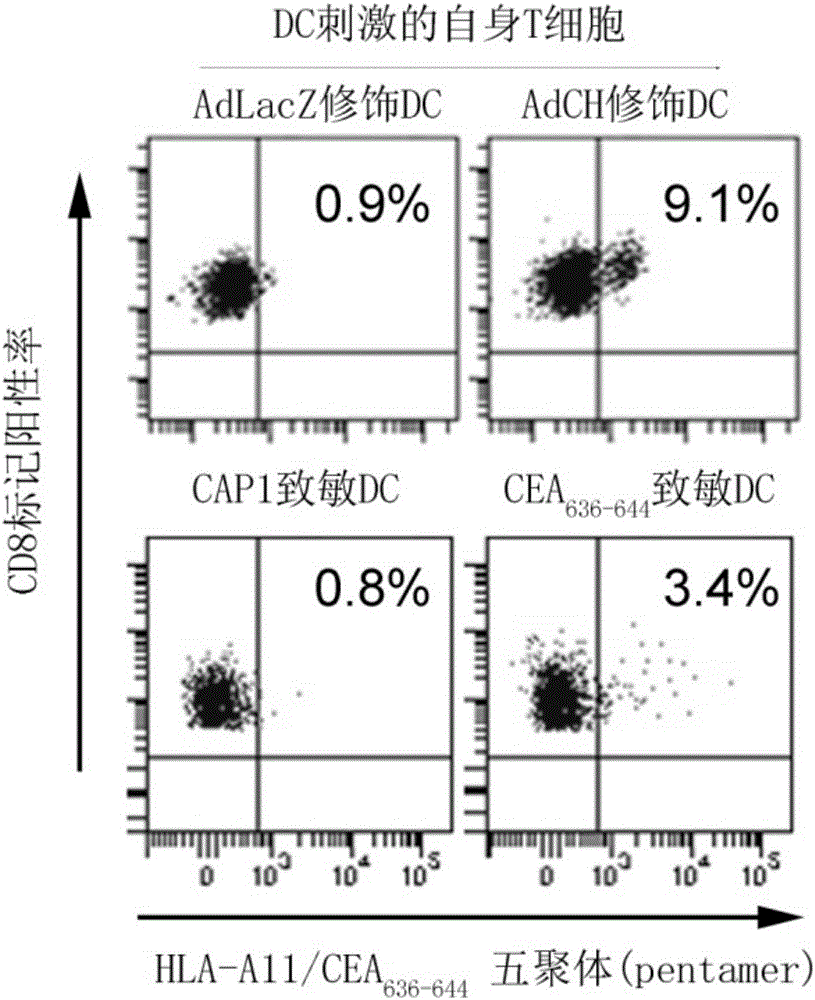
[0048] 2.1 will carry CEA containing 636-644 Peptide CEA 576-669 The recombinant adenovirus (AdCEA 576-669 HSP70L1 (AdCH for short) vector was used to infect human immature MoDC, and after 48 hours, the AdCH-infected human immature MoDC (AdCH-DC) was collected, which was intracellular sensitized DC. As a control, MoDC (AdLacZ-DC) infected with a recombinant adenovirus carrying the LacZ gene was used.
[0049] 2.2 Use CEA directly 636-644 The peptide (50μg / ml) was cultured with human immature MoDC overnight, which...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com