HPLC method for the analysis of related substances of Fimasartan and the use of these impurities as reference standards
A technology for Fimasartan and impurities, which is applied in the field of drug synthesis and can solve problems such as inappropriate determination
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
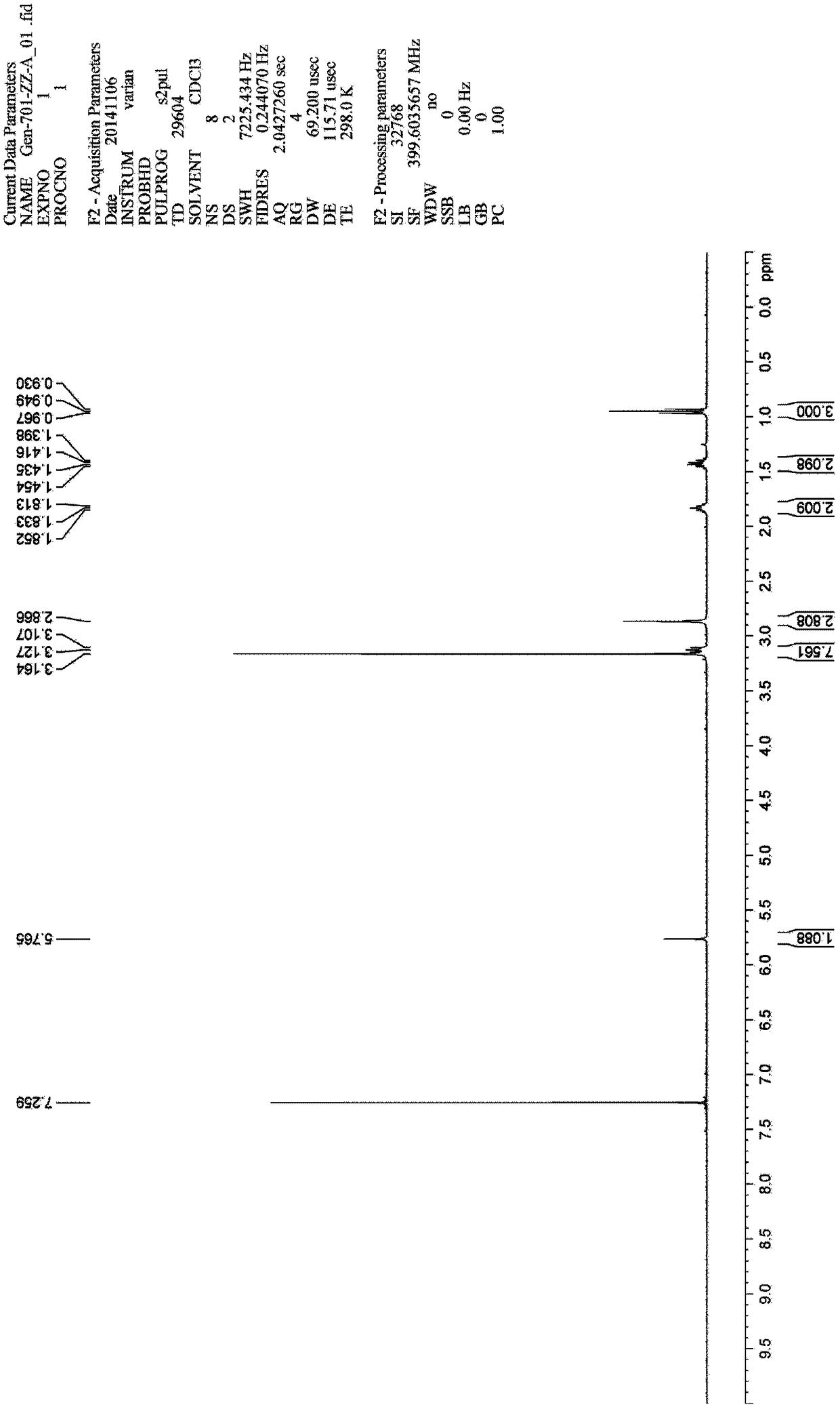
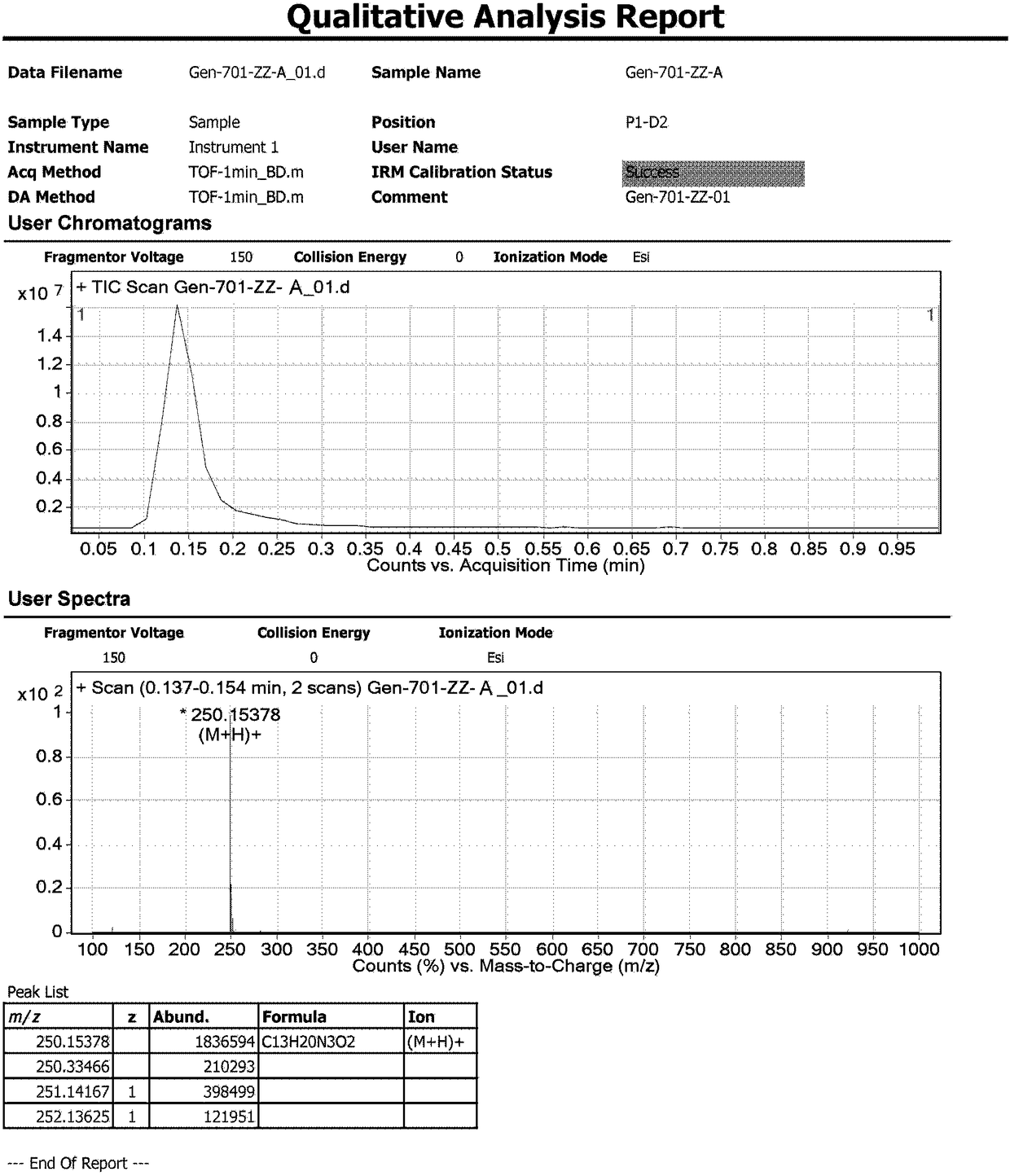
[0123] Example 1: Compound A: Synthesis of (E)-2-(2-butyl-6 methyl-4-carbonylpyrimidinyl-5-(4H)-subunit)-N,N-dimethylacetamide .
[0124]Add 20ml of a mixed solvent of N,N-dimethylformamide and ethyl acetate (volume ratio 1:1) to a 100mL three-necked flask, and add 1.00g of SM1(2-(2-butyl-4-methanol) under stirring Base-6-oxo-1,6-dihydropyrimidin-5-yl)-N,N-dimethylacetamide), nitrogen protection, cooling to 0-10°C, adding 0.48 g of LiH, and continuing to stir for 20-30 Minutes, add 2.08 grams of SM2 (5-(4'-(bromomethyl)-[1,1'-diphenyl]-2-yl)-1-triphenyl-1H-tetrazolium), warming up to Stir at 60° C. for 48 hours; sample TLC (methanol:dichloromethane=1:10) detects that the starting material disappears. After adding 20ml of water, a large amount of solids precipitated, and the stirring was continued for 30 minutes. Filtrate, drain, and blow dry at 40°C for 30 minutes to obtain a solid crude product, which is purified by column chromatography, the elution ratio is: methanol:dic...
Embodiment 2
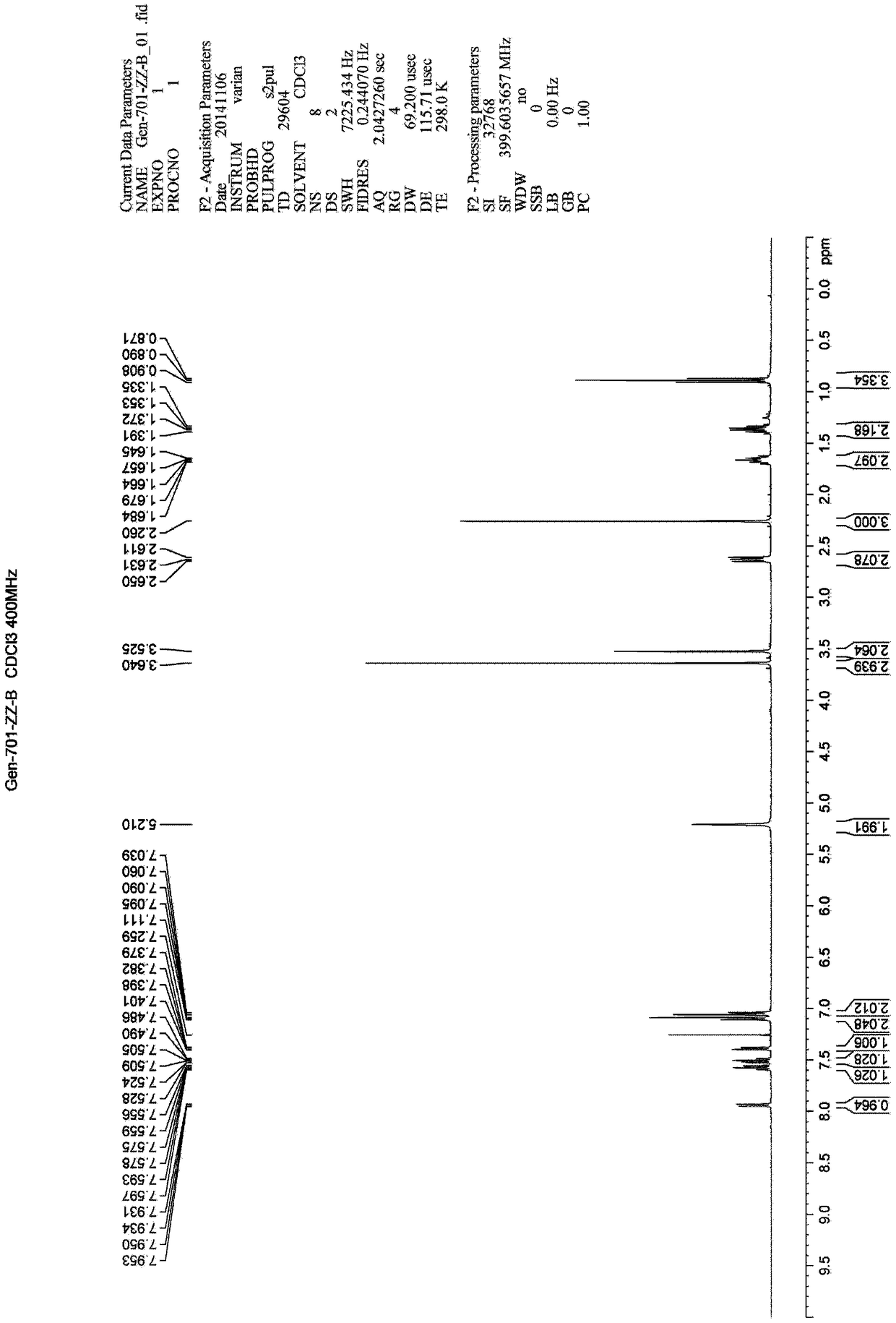
[0125] Example 2: Compound L: 2-(1-((2'-(1H-tetrazol-5-yl)-[1,1'-diphenyl]-4-yl)methyl)-2- Preparation of butyl-4-methyl-6-oxo-1,6-dihydropyrimidin-5-yl)acetylmethyl ester
[0126] Add 20ml of N, N-dimethylformamide and ethyl acetate (volume ratio 1: 1) mixed solvent into the three-necked flask of 100mL, add the impurity of 1.00g SM1 under stirring Shown SM1 impurity), nitrogen protection, cooling to 0~10 ℃, adding 0.48 gram of LiH, continuing to stir for 20~30 minutes, adding 2.08 gram of SM2, heating up to 60 ℃, stirring for 48 hours; sampling TLC detection (methanol: dichloro methane=1:10) the starting material disappeared. After adding 20ml of water, a large amount of solids precipitated, and the stirring was continued for 30 minutes. Filtered, pumped dry, and air-dried at 40°C for 30 minutes to obtain 2.18 g of a solid crude product, which was dissolved in 100 mL of toluene, and 1.57 g of Lawesson's reagent was added. Under nitrogen protection, the reaction solution was...
Embodiment 3
[0127] Example 3: Compound I: 2-(2-butyl-1-((2'-cyano-[1,1'-diphenyl]-4-yl)methyl)-4-methyl-6 Preparation of -Oxy-1,6-dihydropyrimidin-5-yl)-N,N-dimethylthioacetamide
[0128] Add 20ml of a mixed solvent of N,N-dimethylformamide and ethyl acetate (volume ratio 1:1) into a 100mL three-necked flask, add 1.00g of SM1 under stirring, protect with nitrogen, cool to 0-10°C, add 0.30 g of LiH, continue to stir for 20 to 30 minutes, add 1.30 g of 4'-(bromomethyl)-[1,1'-diphenyl]-2-carbonitrile (SM2-impurity-1), heat up to 60 ° C, Stir for 48 hours; sample TLC (methanol:dichloromethane=1:20) detects that the starting material disappears. 15ml of water was added, a large amount of solids were precipitated, and the stirring was continued for 30 minutes. Filtered, pumped dry, and air-dried at 40°C for 30 minutes to obtain 1.41 g of a solid crude product. Dissolve the crude product in 50 mL of toluene, add 1.28 g of Lawesson's reagent, and under nitrogen protection, heat the reaction sol...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com