Synthetic method of 2-formic acid-3-propoxyl-5-methylpyrrole
A technology of methylpyrrole and synthesis method, applied in the direction of organic chemistry, etc., can solve the problems of poor solubility, limitation, harsh synthesis conditions, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
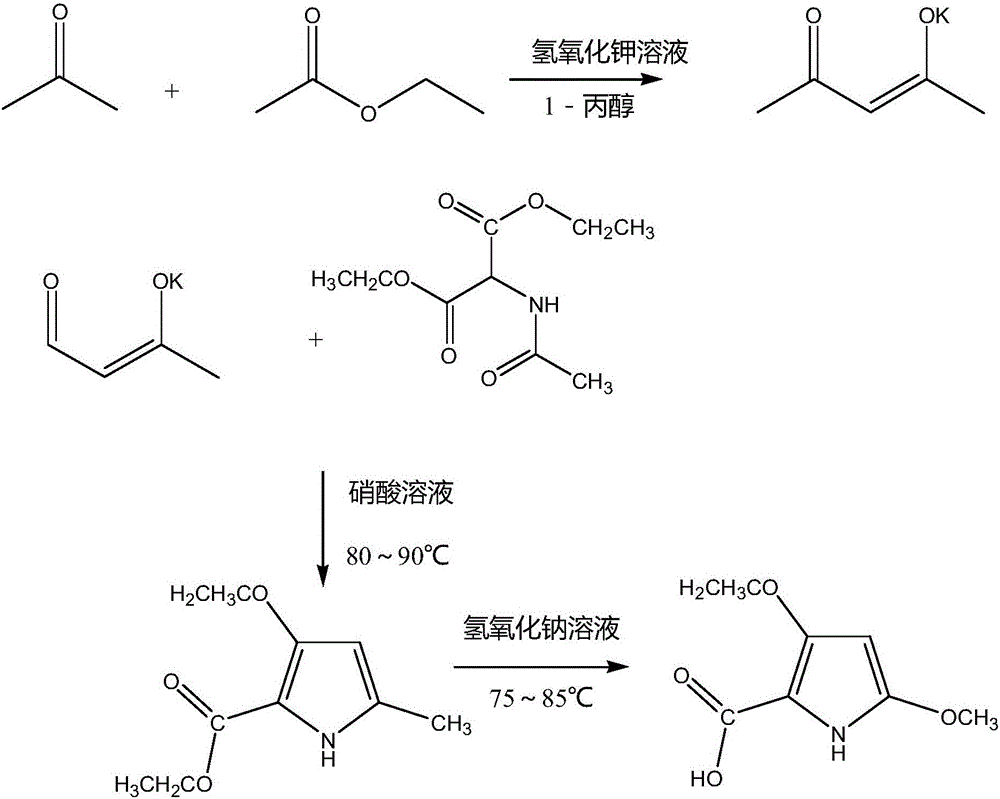
[0015] Add 40mL of ethyl acetate to a 500mL three-necked flask equipped with a thermometer, add 40mL of 1-propanol and 200mL of absolute ethanol into the flask, put it into an ice bath with stirring at 500r / min and cool to -8°C, and keep it Slowly add 20 mL of potassium hydroxide solution with a mass fraction of 25% at high temperature into the flask, continue to stir for 1 hour after the addition, perform suction filtration to remove the organic solvent after the reaction is completed, and wash the filter residue twice with ether before suction filtration and drying. Obtain light yellow solid, namely potassium 4-oxo-2-penten-2-alcohol; add 3 g of above-mentioned potassium 4-oxo-2-penten-2-alkolate in a 250mL three-necked flask, add to it Mix 15mL of 35% nitric acid solution and 30mL of distilled water, mix and stir evenly, pass nitrogen gas into the three-necked flask, and stir for 20 minutes, then stop the flow, heat the temperature to 70°C, add 3g of acetamidopropanediol to ...
example 2
[0017] Add 55mL of ethyl acetate to a 500mL three-necked flask equipped with a thermometer, add 50mL of 1-propanol and 200mL of absolute ethanol into the flask, and put it into an ice bath with stirring at 550r / min to cool to -6°C and keep at this temperature Slowly add 23 mL of potassium hydroxide solution with a mass fraction of 25% into the flask, continue to stir for 1 hour after the addition, and remove the organic solvent by suction filtration after the reaction is completed. Pale yellow solid, which is potassium 4-oxo-2-penten-2-alcohol; add 3.5 g of potassium 4-oxo-2-penten-2-alcohol obtained above into a 250 mL three-necked flask, and add to it 17mL of 35% nitric acid solution and 35mL of distilled water were mixed and stirred evenly, and nitrogen gas was introduced into the three-necked flask, and the reaction was stirred for 25 minutes. Acetate diethyl ester and 13mL of distilled water, while continuing to heat to 85°C for reflux, reflux reaction for 1.5h, after the...
example 3
[0019]Add 70mL of ethyl acetate to a 500mL three-neck flask equipped with a thermometer, add 60mL of 1-propanol and 200mL of absolute ethanol into the flask, put it into an ice bath with stirring at 600r / min, cool to -5°C, and keep at this temperature Slowly add 25 mL of potassium hydroxide solution with a mass fraction of 25% into the flask, continue to stir for 1 hour after the addition, and remove the organic solvent by suction filtration after the reaction is completed. Pale yellow solid, which is potassium 4-oxo-2-penten-2-alcohol; add 4 g of the above-mentioned potassium 4-oxo-2-penten-2-alcohol to a 250 mL three-necked flask, and add 30 mL of potassium 4-oxo-2-penten-2-alcohol Mass fraction 35% nitric acid solution and 40mL distilled water, after mixing and stirring evenly, pass nitrogen gas into the three-necked flask, and stir for 30 minutes, then stop the flow, heat the temperature to 75°C, and add 5g of acetamidomalonic acid into the bottle Diethyl ester and 15mL di...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com