Single domain heavy chain antibody specifically binding to vomitoxin antigen-antibody immune complex and application thereof
A single-domain heavy chain antibody and immune complex technology, applied in anti-fungal/algae/lichen immunoglobulin, application, immunoglobulin, etc., can solve problems such as difficult combination of two antibodies at the same time, difficulty in immune analysis, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1 Affinity panning and identification of single domain heavy chain antibody specifically binding to DON antigen-antibody immune complex
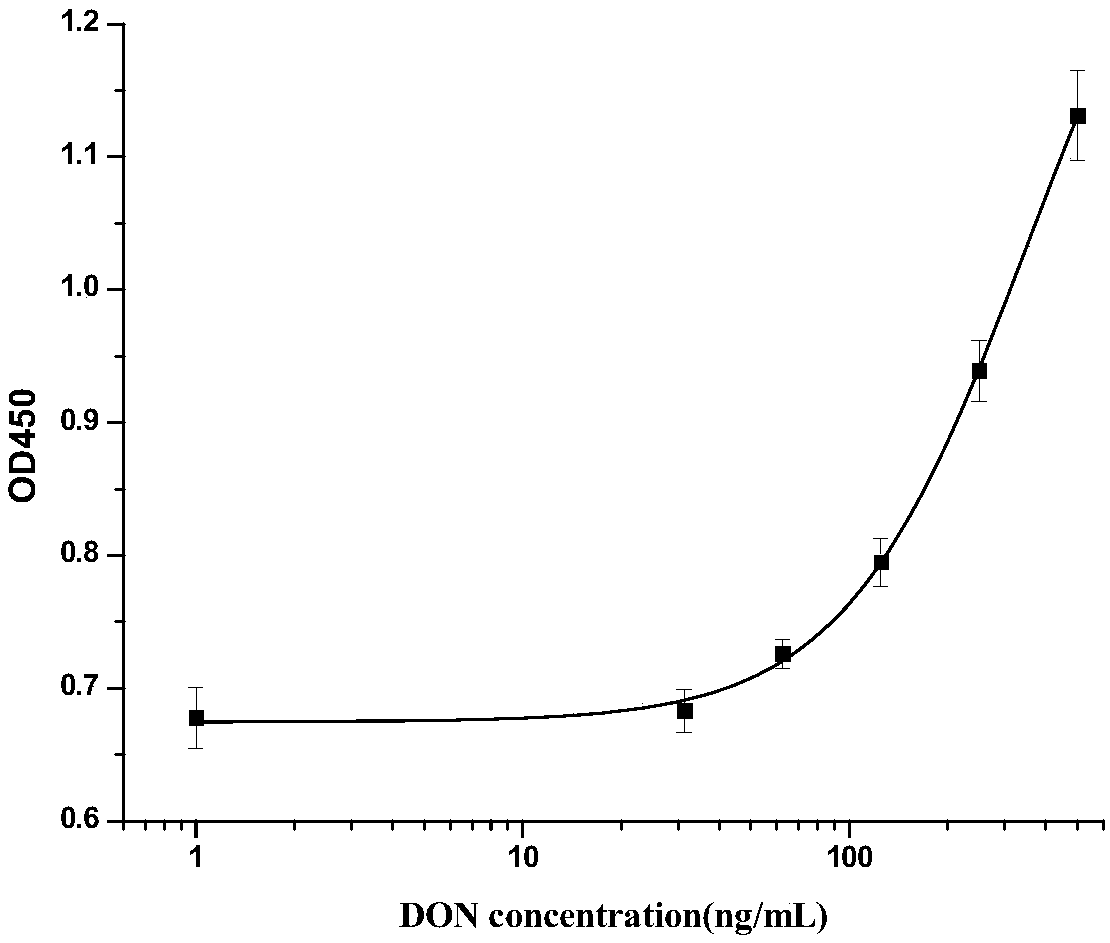
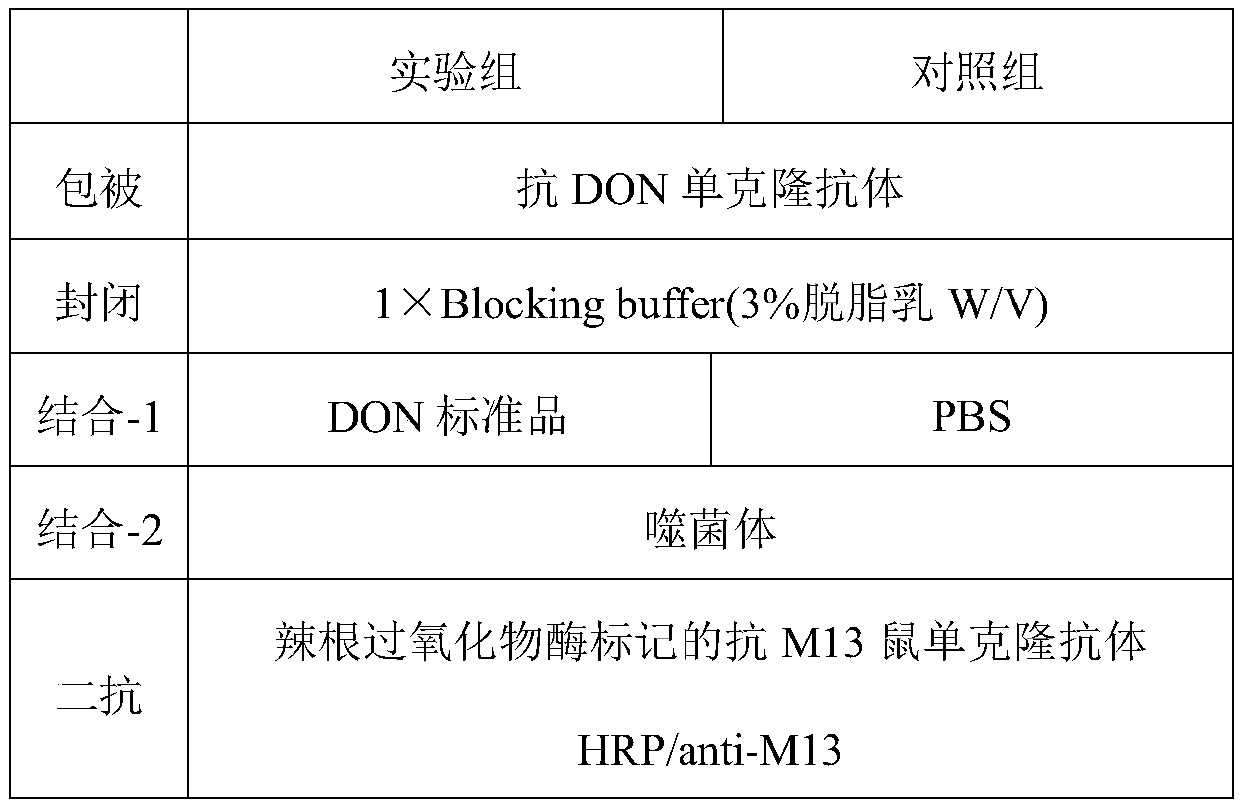
[0027]The single-domain heavy chain antibody against DON antigen-antibody immune complex was screened from camel-derived natural heavy chain antibody library by solid-phase affinity panning. Purify the anti-DON mouse monoclonal antibody ascites with an affinity column to obtain the anti-DON monoclonal antibody; dilute the anti-DON monoclonal antibody to a final concentration of 50 μg / mL with PBS (pH 7.4), coat the wells of the microplate, and store at 4 °C Pack overnight. The next day, after washing 15 times with PBST (10 mM PBS, 0.1% Tween-20 (v / v)), add 1% gelatin to block at 37°C for 1 h; draw up the blocking solution, wash 5 times with PBST, and add 100 μL of DON to the well. Standard product (20ng / mL), incubated at 37°C for 1h to form DON antigen-antibody immune complex; then washed 5 times with PBST, and added 100μL cam...
Embodiment 2
[0035] Example 2 Amplification of Phagemids of Single Domain Heavy Chain Antibody Specifically Binding to DON Antigen-Antibody Complex
[0036] Add the phage displaying the positive single domain heavy chain antibody to 20mL culture inoculated with E.coli TG1, shake and culture at 220rpm at 30°C for 6h; transfer the culture to another centrifuge tube, centrifuge at 4°C and 8000rpm for 15min, Transfer the supernatant to a fresh centrifuge tube, add 1 / 6 volume of PEG / NaCl, let stand at 4°C for 4h, centrifuge at 4°C, 8000rpm for 10min, discard the supernatant; resuspend the phage in 1mL PBS, add 1 / 6 After standing still at 4°C for 1 hour, centrifuge at 10,000 rpm for 10 minutes at 4°C, discard the supernatant, and add 500 μL PBS to resuspend, which is the phage amplification solution.
Embodiment 3
[0037] Example 3 Expression of a single domain heavy chain antibody specifically binding to the DON antigen antibody immune complex in Escherichia coli.
[0038] Phagemid pHEN1 was incompletely digested with restriction endonuclease NotI / NcoI, and the target fragment was recovered from agarose gel.
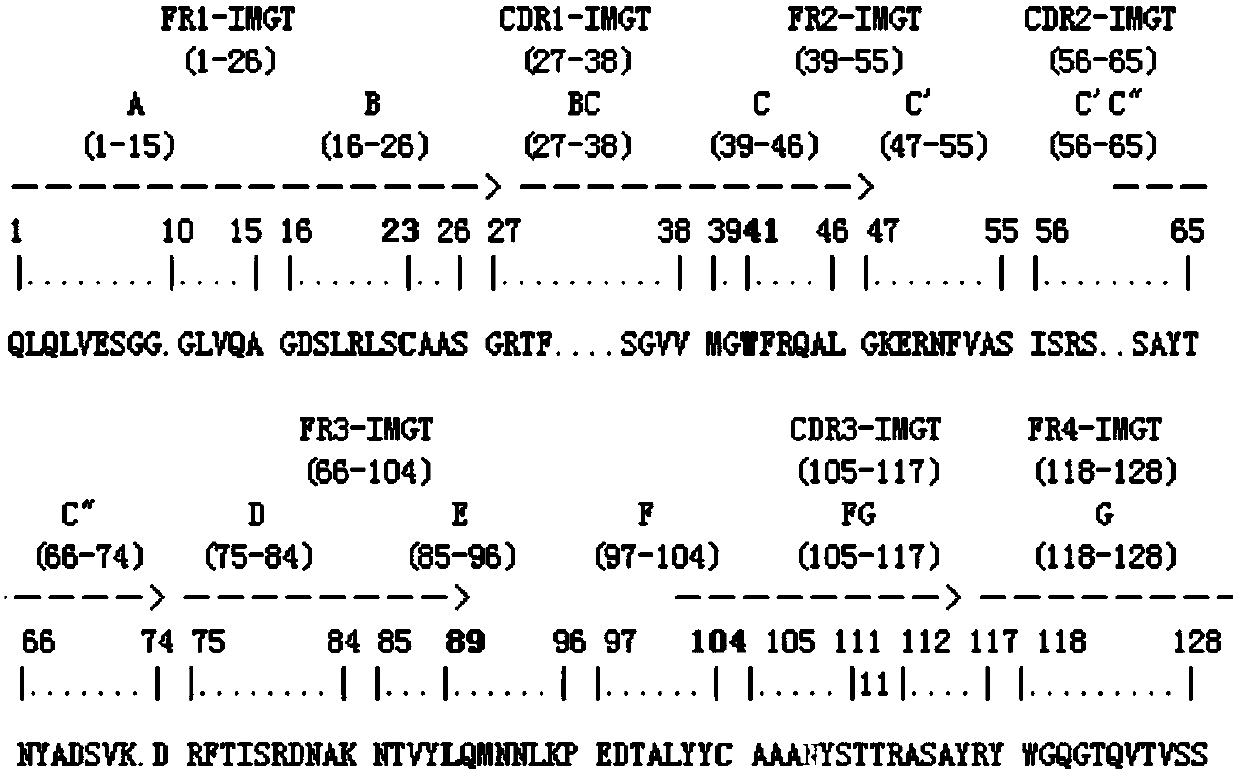
[0039] The obtained single-domain heavy chain antibody double-enzyme-digested gene fragment was cloned into the expression vector pET-25b, and after sequencing verification, it was named pET25b-DON.
[0040] The recombinant plasmid pET25b-DON was transformed into Escherichia coli Rosetta and induced to express. Pick a single colony and inoculate it into 5mL liquid LB / Amp medium, and culture it on a shaker at 37°C and 200rpm for 10h; inoculate the above culture solution with 1% inoculum size into 50mL liquid LB medium, shake it at 37°C and 200rpm After culturing to an OD of 0.5, a final concentration of 0.05 mM IPTG was added to induce culture at 30° C. and 180 rpm on a shaking ta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com