A kind of benzothiazole 2-acetonitrile derivative and its application
A technology of benzothiazole and its derivatives, which is applied in the application field of benzothiazole 2-acetonitrile, can solve the problems of easy quenching and no aggregation-induced luminescent effect, and achieve good biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] 1. Preparation of benzothiazole 2-acetonitrile derivatives
[0021] Add 1.77g (10mmol) 4-(diphenylamine) benzaldehyde, 1.74g (10mmol) benzothiazole 2-acetonitrile-2-acetonitrile and 0.77g (10mmol) ammonium acetate in a 50mL flask, and then add 20mL of absolute ethanol. After reacting overnight at room temperature, the precipitate was filtered and recrystallized in ethanol to obtain 2.36 g of an orange-yellow solid. Yield 71%.
[0022] 2. Compound Characterization
[0023] 1 H NMR (400MHz, CDCl 3 )δ (ppm): 7.98 (s, 1H), 7.92 (d, 1H), 7.87 (d, 2H), 7.77 (d, 1H), 7.39 (t, 1H), 7.27 (t, 1H), 6.61 ( d, 2H), 3.36(dd, 4H), 1.64(s, IH), 1.14(t, 6H).
[0024] 13 C NMR (100MHz, CDCl 3 )δ (ppm): 163.88, 152.83, 149.82, 145.86, 132.42, 125.49, 123.96, 121.73, 120.42, 118.58, 110.28, 95.88, 43.68, 28.68, 11.57.
[0025] IR (v-1, KBr): 3396, 2973, 2357, 2208, 1590, 1569, 1515, 1468, 1405, 1352, 1268, 1179, 1146, 1069, 986, 819, 762, 724, 670, 584, 519 .
[0026] HR-MS(ESI):...
Embodiment 2
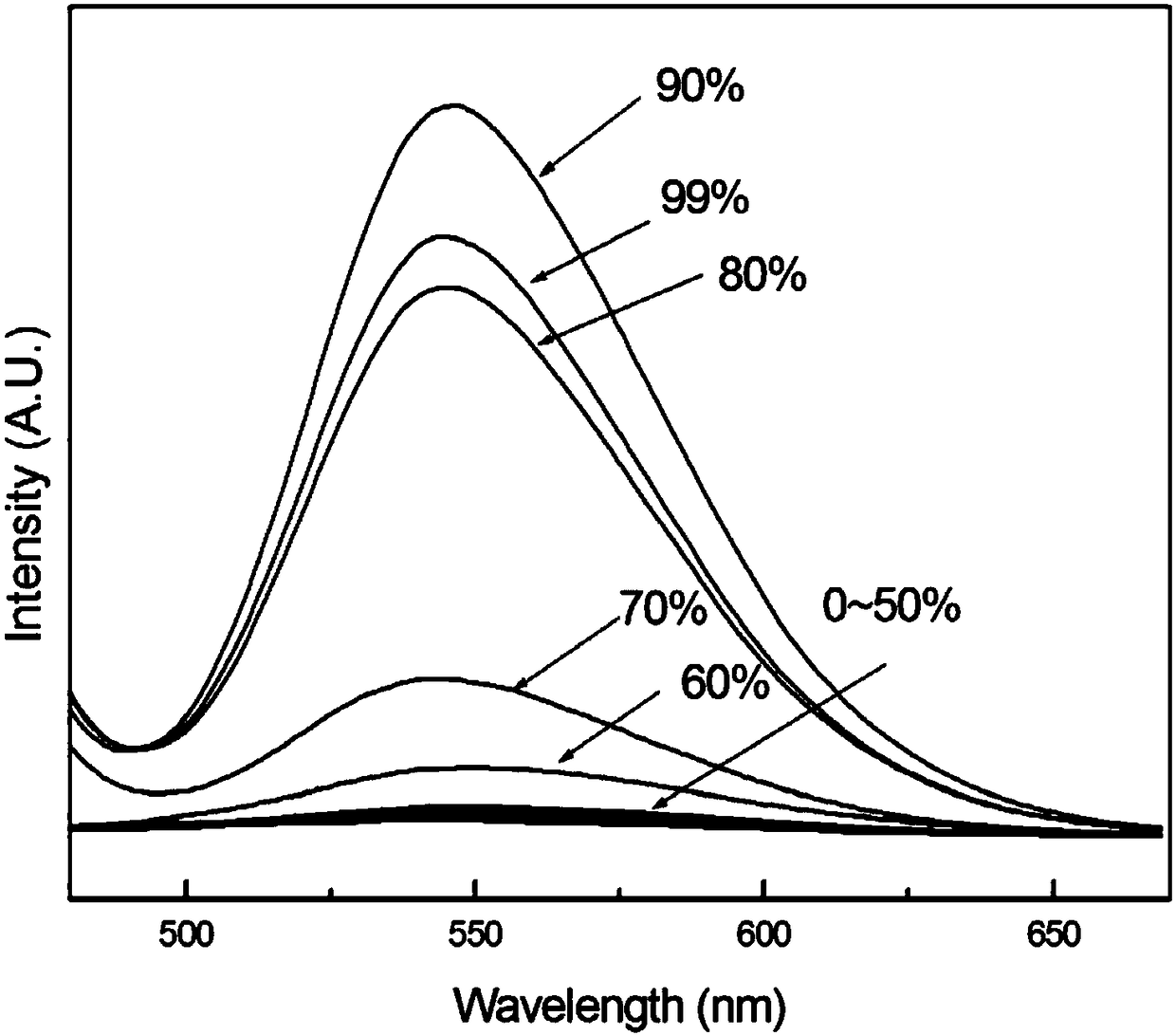
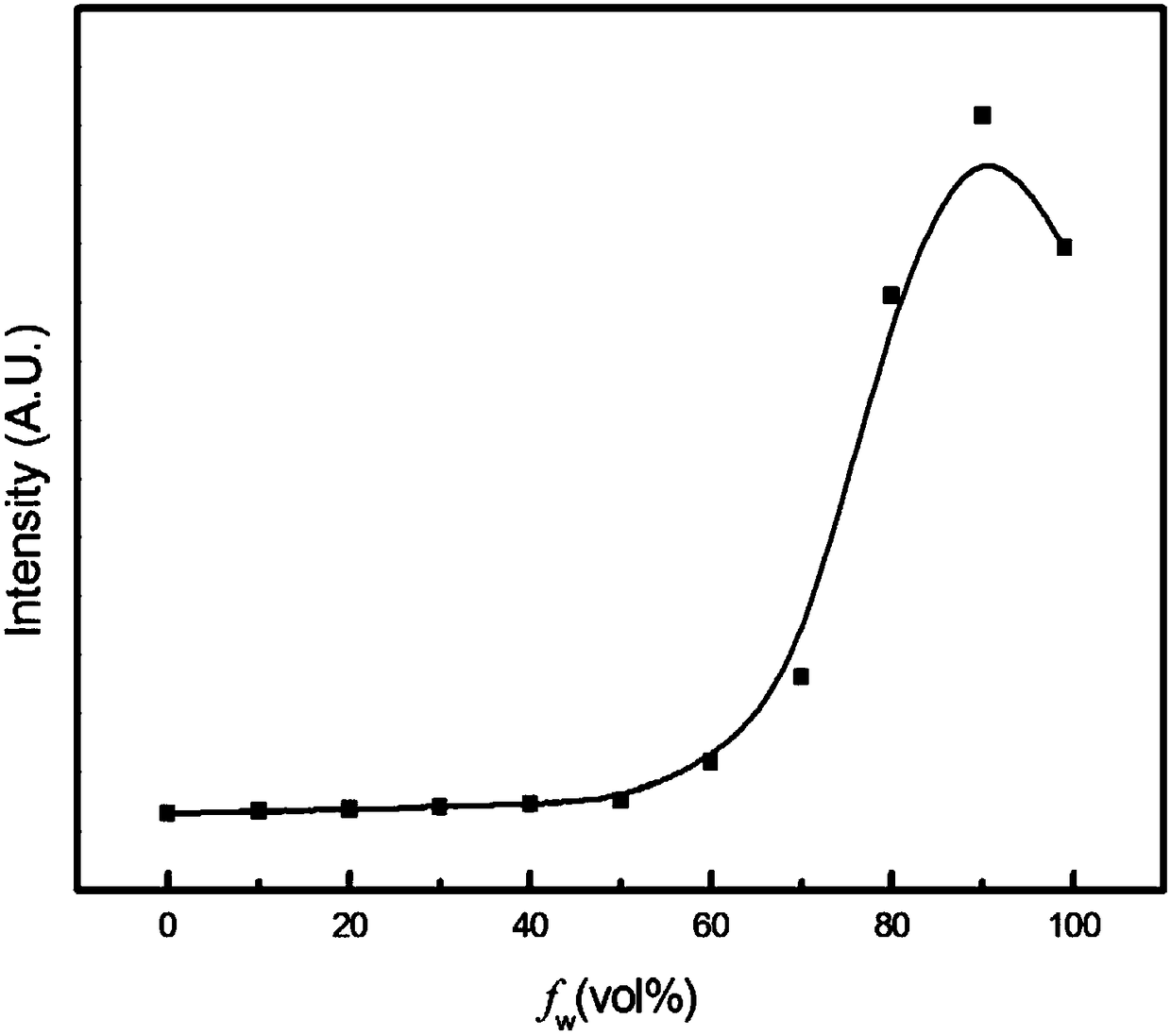
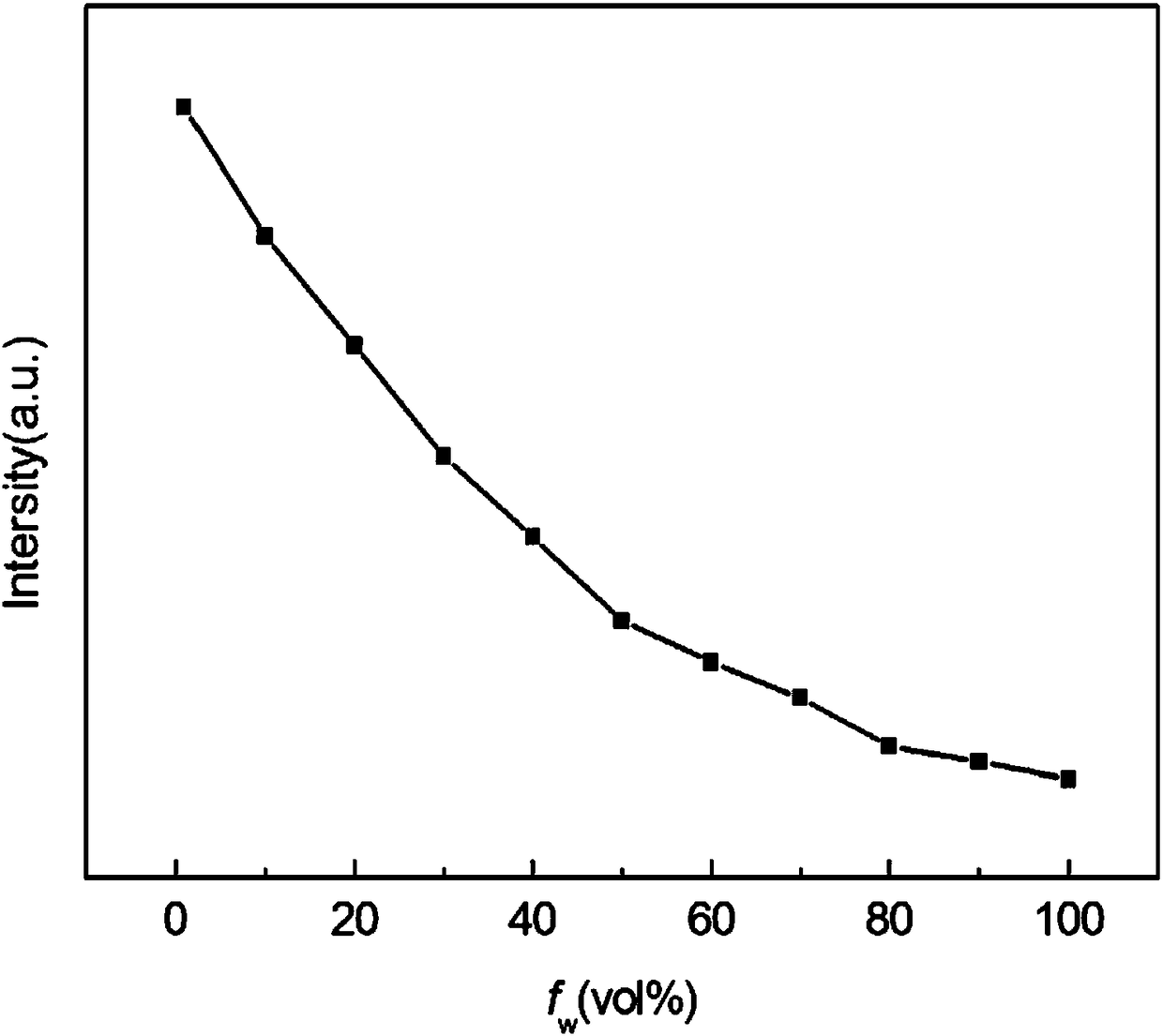
[0029] Embodiment 2 (fluorescent properties of benzothiazole 2-acetonitrile derivatives)
[0030] Prepare acetonitrile solution of benzothiazole 2-acetonitrile derivative with a concentration of 5mM, take 10 μL of benzothiazole 2-acetonitrile derivative acetonitrile solution, add it to a 10mL volumetric flask, add 1, 2, 3, 4, 5, 6, 7 , 8, 9mL of distilled water, and then add acetonitrile to adjust the volume of the solution to 10mL to obtain a water / acetonitrile solution (9 / 1, v / v) of benzothiazole 2-acetonitrile derivatives with a concentration of 5 μM, and a benzothiazole with a concentration of 5 μM 2-acetonitrile derivative water / acetonitrile solution (8 / 2, v / v), the concentration of benzothiazole 2-acetonitrile derivative water / acetonitrile solution (7 / 3, v / v), the concentration of 1 μM benzene Benzothiazole 2-acetonitrile derivative water / acetonitrile solution (6 / 4, v / v), the concentration is 5 μM Benzothiazole 2-acetonitrile derivative water / acetonitrile solution (5 / 5, ...
Embodiment 3
[0039] Embodiment 3 (cell culture and fluorescence imaging)
[0040] HeLa cells in the logarithmic growth phase were inoculated into a 6-well plate, cultured overnight, replaced with 1640 culture medium containing 10 μM of the benzothiazole 2-acetonitrile derivative obtained in Example 1, and continued to culture for 30 minutes, then phosphate buffered saline The 6-well plate was washed three times with liquid to remove excess benzothiazole 2-acetonitrile derivative. After the cells were continued to be cultured in 1640 culture medium with DAPI (10 g / ml) for 30 min, the 6-well plate was washed 3 times with phosphate buffered saline to remove excess DAPI. Cells were observed under a confocal laser microscope. Shows yellow-green fluorescence in the cytoplasm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com