Application of arylhydrazide compound in treatment of acute myocardial ischemic coronary heart disease
A technology for acute myocardial ischemia and coronary heart disease, applied in the field of medicine, to prolong the hypoxia tolerance time and increase the serum NO content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
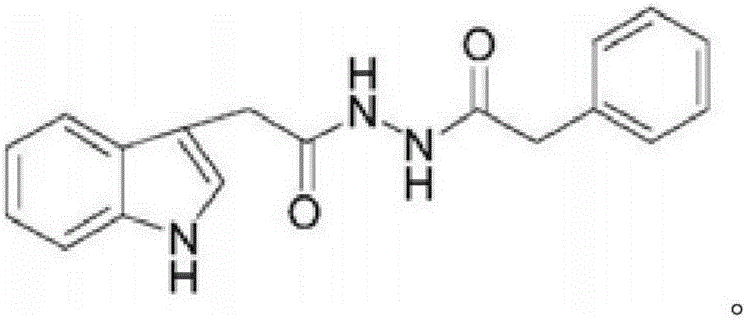
[0011] Example 1: Preparation of N-(2-(1H-indol-3-yl)acetyl)phenylacetylhydrazide
[0012] (1) Add 8.8g (50mmol) of indole acetic acid (50mmol), methanol (60mL), and concentrated sulfuric acid (3mL) to a 500ml round bottom flask, and react at 70°C for 1-3 hours. water (50mL), separate the organic phase, extract the aqueous phase with ethyl acetate (3×20mL), combine the organic phases, wash with saturated sodium bicarbonate solution and water successively, dry over anhydrous sodium sulfate, and concentrate under reduced pressure to obtain indole Crude methyl acetate.
[0013] (2) Add 9.46g (48mmol) of methyl indole acetate, ethylene glycol methyl ether (40mL), and 5mL of hydrazine hydrate into a 500ml round-bottomed flask, heat and reflux at 115°C for about 20 hours, thin-layer chromatography (TLC) After detecting the disappearance of the raw material point, stop the reaction, cool to room temperature, add water (50 mL), stand still to precipitate a white solid, obtain the cru...
Embodiment 2
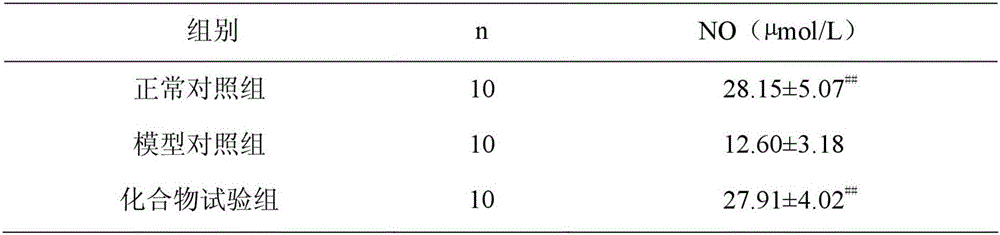
[0016] Example 2: Experimental study on the influence of N-(2-(1H-indol-3-yl)acetyl)phenylacetylhydrazide on serum NO content in rats with acute myocardial ischemia
[0017] Thirty male healthy Wistar rats, weighing 220-250 g, were randomly divided into the following three groups: normal control group, model control group, and compound test group, with 10 rats in each group. Based on the rat body weight, rats in the compound test group were intravenously injected with N-(2-(1H-indol-3-yl)acetyl)phenylacetylhydrazide 20mg / kg, and the model control group and the normal control group were intravenously injected, etc. volume of saline. Each group was injected once a day between 9:00-10:00 am for 7 consecutive days. Ten minutes after the injection on the 5th, 6th, and 7th day, except the normal control group, the rats in the other two groups were intraperitoneally injected with isoproterenol 2 mg / kg to create a rat model of acute myocardial ischemia. 24 hours after the last injec...
Embodiment 3
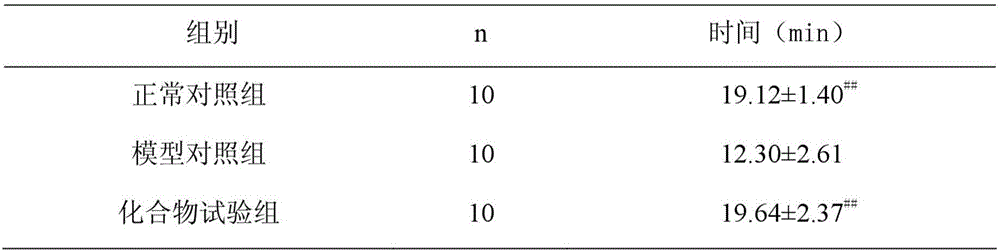
[0022] Example 3: Experimental study on the influence of N-(2-(1H-indol-3-yl)acetyl)phenylacetylhydrazine on the hypoxia tolerance time of acute myocardial ischemia mice
[0023] 50 healthy Kunming mice, weighing 18-22 g, half male and half male, were randomly divided into the following three groups: normal control group, model control group, and compound test group, with 10 mice in each group. Based on the body weight of the mice, the mice in the compound test group were intravenously injected with N-(2-(1H-indol-3-yl)acetyl)phenylacetylhydrazide 32 mg / kg, and the model control group and the normal control group were injected intravenously, etc. volume of saline. Each group was injected once a day between 9:00-10:00 am for 8 consecutive days. Ten minutes after the injection on the eighth day, except for the normal control group, 2 mg / kg of isoproterenol was subcutaneously injected into mice in each group to create a mouse model of acute myocardial ischemia. After each group...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap