A method for highly synchronized somatic embryogenesis and plant regeneration of pine wood nematode-resistant Pinus pine
A technology for pine wood nematode disease and somatic embryos, applied in the field of highly synchronized anti-pine wood nematode disease pine somatic embryogenesis and plant regeneration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] ABA concentration (10, 15, 20mg / L) and PEG (polyethylene glycol) 8000 concentration (100, 140, 180g / L) were subjected to a two-factor randomized block test. will be 22 # The embryogenic suspensor mass (ESM) of -1 was transferred to the designed medium (basic medium LP, additionally inositol 8g / L, maltose 60g / L, MES 250mg / L, CH 500mg / L , VC 10mg / L, glutamine 450mg / L, activated carbon (AC) 2.0g / L, plant gel 3g / L, pH 5.8), 30 pieces of ESM per treatment, repeated 3 times, real-time observation of somatic embryo maturation Happening. Medium. The medium was sterilized by autoclaving at 121°C for 20min.
[0023] Induction, proliferation and maturation experiments of somatic cell embryos of disease-resistant Scots pine were cultured in the dark in a tissue culture room with a culture temperature set at 23±2°C, and observed once every two weeks. The number of normal somatic embryos is the number of somatic embryos formed per gram of callus (piece / g) = the number of normal s...
Embodiment 2
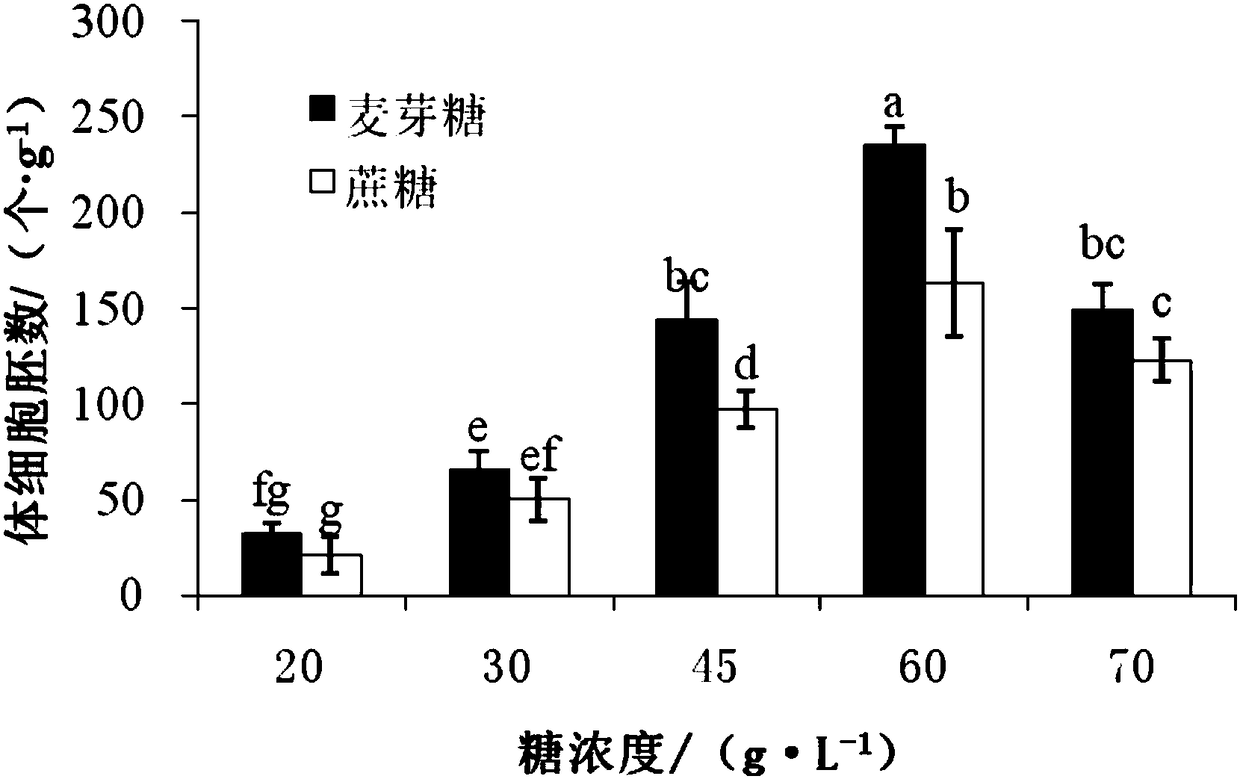
[0029] will be 22 #-1 ESM was transferred to LP as the basic medium, adding different types of sugar (maltose, sucrose) and different concentrations (20, 30, 45, 60, 70g / L), 30 pieces of ESM per treatment, repeated 3 times , real-time observation of somatic embryo maturation. The medium was supplemented with ABA 15mg / L, PEG8000 140g / L, inositol 8g / L, MES 250mg / L, CH 500mg / L, VC 10mg / L, glutamine 450mg / L, activated carbon (AC) 2.0g / L, Plant gel 3g / L, pH5.8. The medium was sterilized by autoclaving at 121°C for 20min. Somatic embryo culture and measurement are the same as in Example 1.
[0030] The results showed that the effects of sugar type and concentration on the maturation of somatic embryos of resistant Pinus spp. were significantly different, and maltose was more effective than sucrose in increasing the number of somatic embryos of resistant Scots pine ( figure 1 ). When the concentration of maltose was 60g / L, the number of somatic embryos of the disease-resistant P...
Embodiment 3
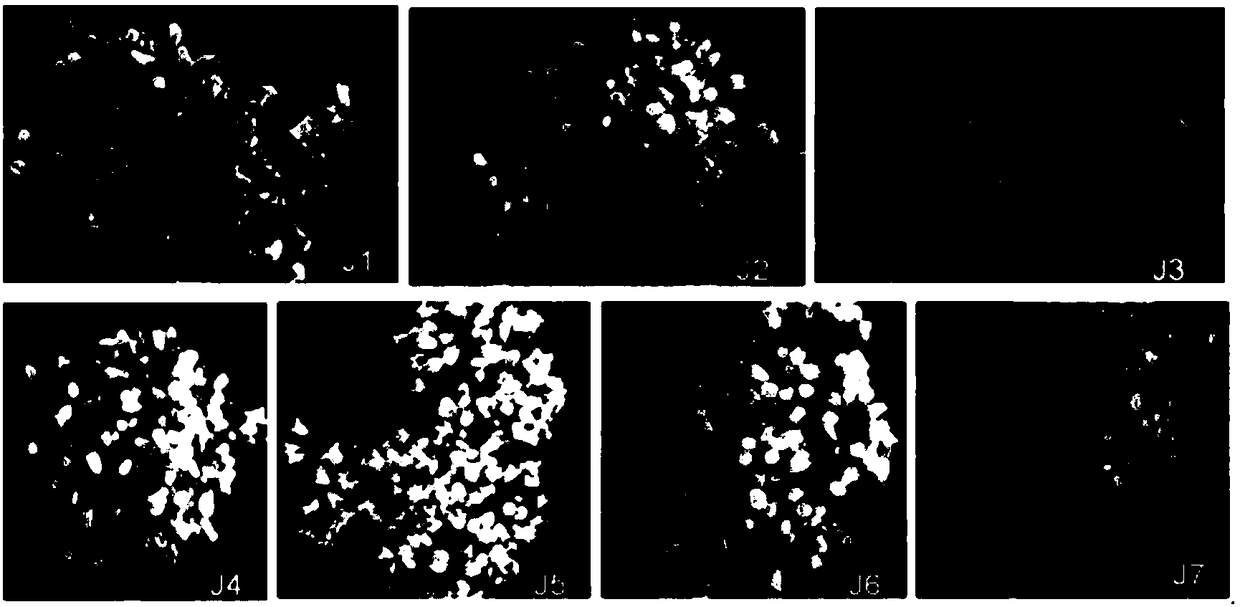
[0032] will be 22 # -1 ESM was transferred to LP basic medium, adding inositol 0, 2, 4, 6, 8, 10, 16g / L, 30 ESMs per treatment, repeated 3 times, and observed the maturation of somatic embryos in real time. Added ABA 15mg / L, PEG8000 140g / L, MES250mg / L, CH 500mg / L, VC 10mg / L, glutamine 450mg / L, activated carbon (AC) 2.0g / L, plant gel 3g / L, pH5.8. The medium was sterilized by autoclaving at 121°C for 20min. Somatic embryo culture and measurement are the same as in Example 1.
[0033] the result shows( figure 2 ), in the absence of inositol, although the resistant pine can produce cotyledon embryos, the cotyledon embryo head is enlarged, the embryo stalk is short and even cannot develop normally, the embryo body is short and thick, the color is yellowish, and the growth state is poor, all of which are deformed embryo; and the color of the callus is brownish, the structure is gradually compacted, and the growth status obviously declines ( figure 2 -J1). When adding inosito...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com