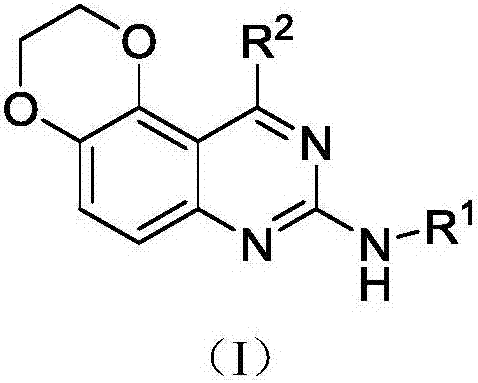
Quinazoline heterocyclic compounds as well as preparation method and application thereof
An amine compound, quinazoline technology, applied in the field of medicinal chemistry, can solve the problems of few chemical types, limited clinical application, low selectivity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0079] N 8 -Methyl-N 10 - Preparation of p-tolyl-2,3-dihydro-[1,4]dioxane[2,3-f]quinazoline-8,10-diamine
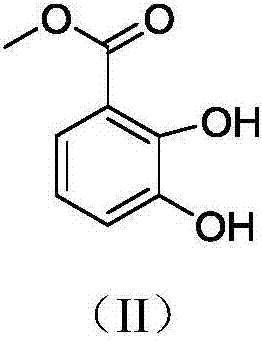
[0080] (a) Preparation of 2,3-dihydroxy-methyl benzoate
[0081]
[0082] The starting material 2,3-dihydroxy-benzoic acid (15 g, 97.3 mmol) was dissolved in methanol (150 mL) and cooled to 0 °C with stirring. Concentrated sulfuric acid (9 mL) was added dropwise to the solution. The reaction mixture was heated to reflux for 8 hours, and the reaction was completed as monitored by TLC, showing a brownish yellow color. The methanol solvent was evaporated to give a light brown oil. Add ethyl acetate and saturated NaHCO 3 solution until effervescence ceases. The aqueous phase was extracted with ethyl acetate (3x150 mL), dried over anhydrous sodium sulfate, and the solvent was evaporated under reduced pressure to obtain a gray solid. Yield 95%.
[0083] 1 H NMR (400MHz, CDCl 3 )δ=10.90(s,1H),7.35(dd,=8.1,1.5Hz,1H),7.10(dd,J=7.9,1.4Hz,1H),6.78(t,J=8.0Hz,1H),5.81 (s...
Embodiment 2
[0124] N 10 - Preparation of p-tolyl-2,3-dihydro-[1,4]dioxane[2,3-f]quinazoline-8,10-diamine
[0125] Steps (a), (b), (c), (d), (e), (f), (g), (h), (i) are the same as in Example 1.
[0126] (j)N 10 - Preparation of p-tolyl-2,3-dihydro-[1,4]dioxane[2,3-f]quinazoline-8,10-diamine
[0127]
[0128] The intermediate 8-chloro-N-p-tolyl-2,3-dihydro-[1,4]dioxane[2,3-f]quinazoline-10- The amine was reacted with ammonia (38%) (5 eq), ethanol (2 mL) and tetrahydrofuran (2 mL). The progress of the reaction was monitored by TLC until no starting material was observed. After column chromatography, a yellow solid was obtained. Yield: 32%.
[0129] 1 H NMR (400MHz, CDCl 3 )δ=9.36(s,1H),7.53(d,J=8.4Hz,2H),7.13(d,J=8.9Hz,3H),6.98(d,J=9.0Hz,1H),4.91(s, 2H), 4.48–4.44(m,2H), 4.32–4.29(m,2H), 2.31(s,3H).
[0130] MS m / z:309.2[M+H] +
Embodiment 3
[0132] N 8 -Methyl-N 10 Preparation of -phenyl-2,3-dihydro-[1,4]dioxane[2,3-f]quinazoline-8,10-diamine
[0133] Steps (a), (b), (c), (d), (e), (f), (g), (h), are the same as in Example 1.
[0134] (i) Preparation of 8-chloro-N-phenyl-2,3-dihydro-[1,4]dioxane[2,3-f]quinazolin-10-amine
[0135]
[0136] Will dissolve in THF / H 2 8,10-Dichloro-2,3-dihydro-[1,4]dioxane[2,3-f]quinazoline (51.2mg, 0.2mmol) in O (2ml / 1ml), aniline ( 1.1 eq) and potassium acetate (25.4 mg, 0.26 mmol) were stirred at room temperature for 4 hours. Ethyl acetate was added to the mixture for extraction, the organic layer was collected, dried over anhydrous sodium sulfate, and the excess solvent was distilled off under reduced pressure to obtain a yellow solid. Yield: 98%.
[0137] MS m / z:314.1[M+H] +
[0138] (j)N 8 -Methyl-N 10 Preparation of -phenyl-2,3-dihydro-[1,4]dioxane[2,3-f]quinazoline-8,10-diamine
[0139]
[0140] The intermediate 8-chloro-N-phenyl-2,3-dihydro-[1,4]dioxane[2,3-f]...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap