Application of polypeptide c2orf40mpf in the preparation of antitumor drugs
A technology of C2ORF40MPF and drugs, applied in the field of biomedicine, can solve the problems of different therapeutic targets and mechanisms of action, and achieve the effect of inhibiting proliferation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1: Synthesis of polypeptide C2ORF40MPF
[0038] The amino acid sequence of the polypeptide C2ORF40MPF of the present invention is: Ser-Pro-Tyr-Gly-Phe-Arg-His-Gly-Ala-Ser-Val-Asn-Tyr-Asp-Asp-Tyr (SEQ ID NO. 1), commissioned Synpeptide company conducts peptide synthesis.
[0039] In order to compare the actual effect of the tumor suppressor polypeptide C2ORF40MPF designed in the present invention, we scrambled its amino acid sequence and commissioned a company to synthesize it to obtain Scrambled C2ORF40 mimic peptide (ScrC2ORF40), whose sequence is Asp-Ala-Phe-Tyr-Tyr- Arg-Asn-Gly-Asp-His-Tyr-Pro-Val-Ser-Gly-Ser.
Embodiment 2
[0040] Example 2: MTT experiment
[0041] 1. Experimental method:
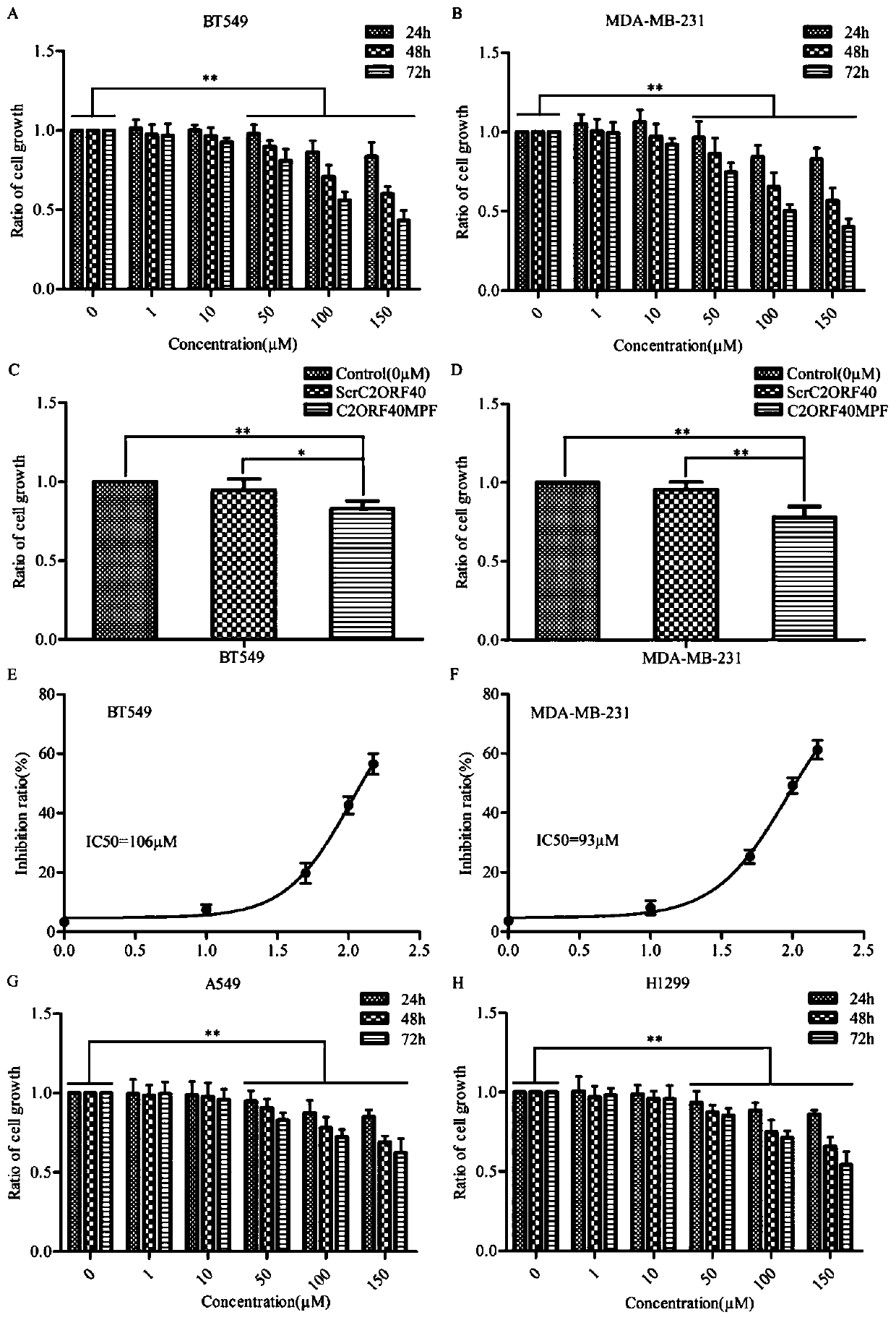
[0042] at 37°C, 5% CO 2 Breast cancer cell lines BT549, MDAMB231, and lung cancer cell lines A549, H1299 were cultured in a cell incubator with saturated humidity. When the cell density reached about 70%, the cells were washed once with PBS, digested with trypsin, and then added to the culture medium to suspend the cells and centrifuged. Collect the cells, remove the supernatant, add the culture medium to resuspend the cells and count the cells. 3 The density per well was inoculated into 96-well plates; after culturing for 24 hours, the medium was replaced, and the medium containing different doses of polypeptides (polypeptides C2ORF40MPF and ScrC2ORF40 prepared in Example 1) were added respectively, and the culture was continued; Add 10 μl of 5 mg / ml MTT solution to each well, continue to incubate for 4 h to remove the supernatant from each well, then add 150 μl of DMSO solution, place it on a shaker and sh...
Embodiment 3
[0048] Example 3: Clone formation experiments
[0049] 1. Experimental method
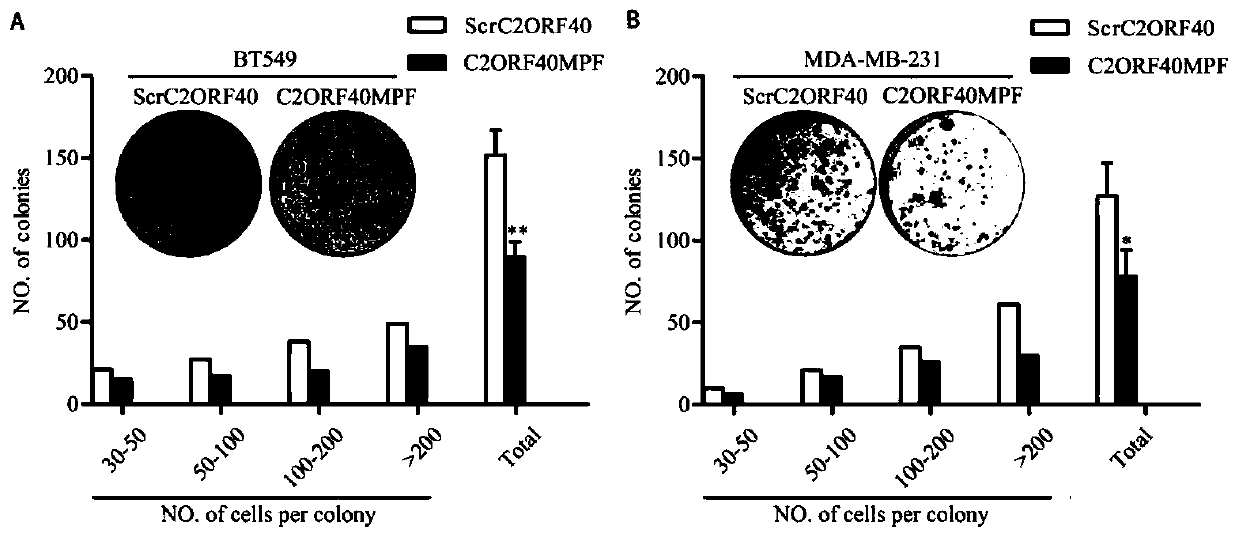
[0050] BT549 and MDAMB231 cells were inoculated into 6cm dishes, 500 cells / dish, and the medium was replaced after culturing for 96 hours, and the culture medium containing 100 μM ScrC2ORF40 or 100 μM C2ORF40MPF polypeptide was added, and the culture was continued for about 2 weeks, and the culture was terminated. The culture medium was discarded, washed twice with PBS, and 5 ml of methanol was added to fix the cells. After 15 minutes, the fixative was removed, and 1 ml of Giemsa was added to stain for 30 minutes. Then, gently wash off the dye solution with running water, dry the petri dish in the air, and finally count the number of clones under the microscope, according to the number of cells per clone: 30-50, 50-100, 100-200, >200 The number of clones was counted separately.
[0051] 2. Experimental results:
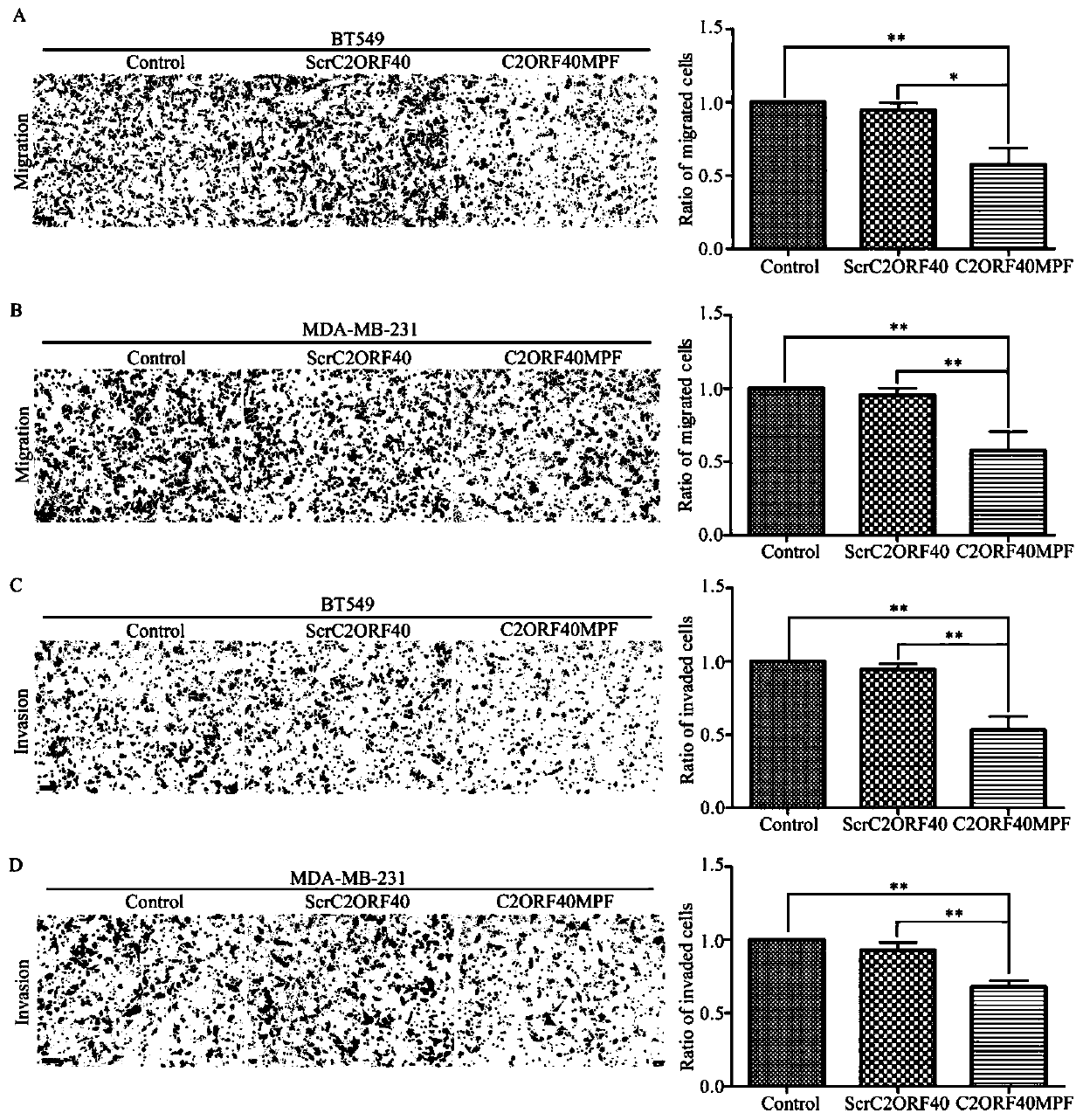
[0052] The experimental results are as figure 2 As shown, in the figure, A and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com