A kind of preparation method of valsartan hydrochlorothiazide capsule
A technology for hydrochlorothiazide and hydrochlorothiazide, which is applied in the field of preparation of valsartan hydrochlorothiazide capsules, can solve the problems of complex preparation process, high equipment requirements, large dust and the like, and achieves the effects of simple process and low preparation cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
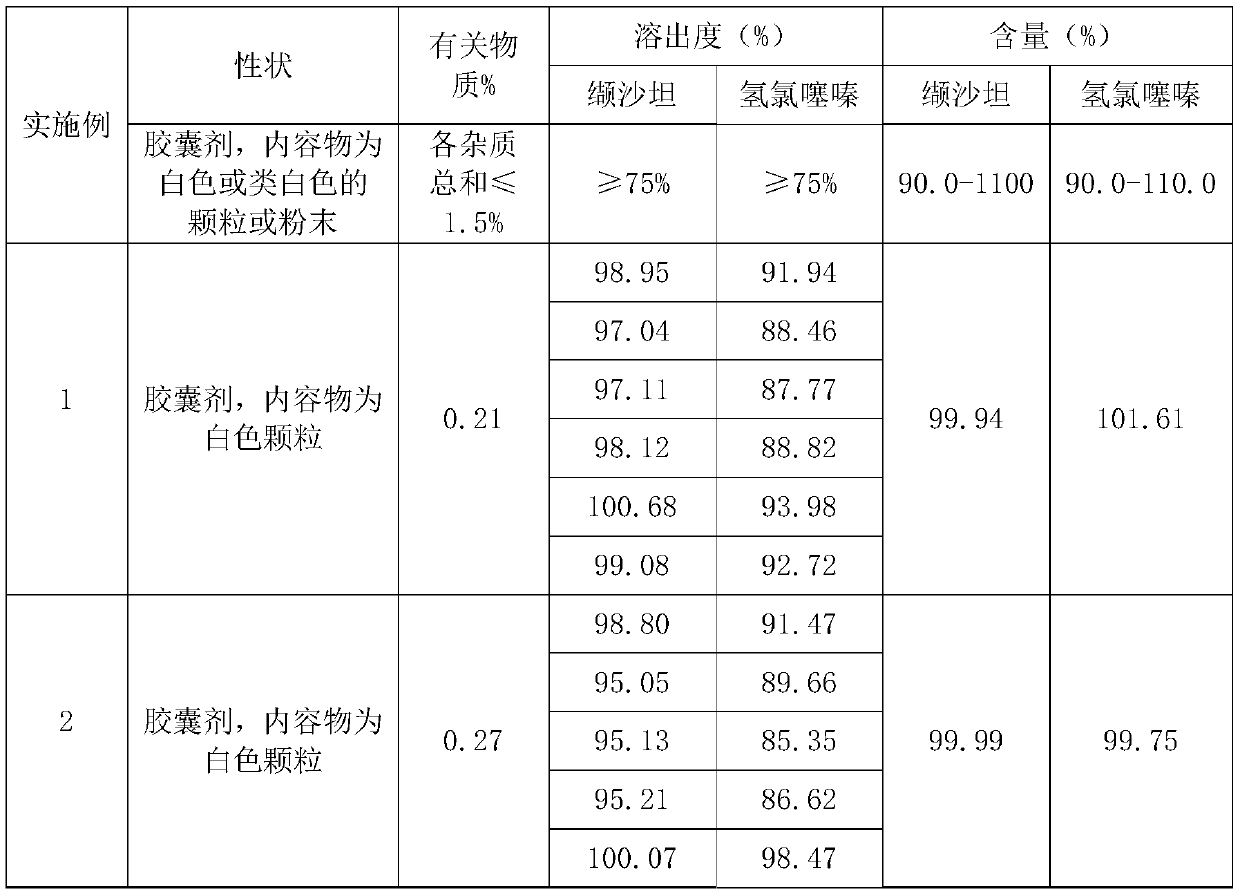
Embodiment 1
[0024] Embodiment 1 (500,000 grains):
[0025] A preparation method of valsartan hydrochlorothiazide capsules, comprising the following steps:
[0026] S1. Weigh valsartan, hydrochlorothiazide, disintegrant, cosolvent, filler, lubricant, and binder according to the proportion, wherein the disintegrator is sodium carboxymethyl starch, and the filler is microcrystalline Cellulose and cornstarch, described solubilizer is sodium lauryl sulfate, described lubricant is magnesium stearate, described binding agent is povidone K30;
[0027] S2, preparing a binder solution, adding the binder weighed in proportion to purified water to prepare a binder solution with a weight ratio of 5-10%, stirring evenly for later use;
[0028] S3. Mix and pulverize valsartan, 50% disintegrant, and filler through an 80-mesh sieve to obtain a mixed material, mix and pulverize hydrochlorothiazide and a cosolvent through an 80-mesh sieve to obtain hydrochlorothiazide powder;
[0029] S4. Add the mixed ma...
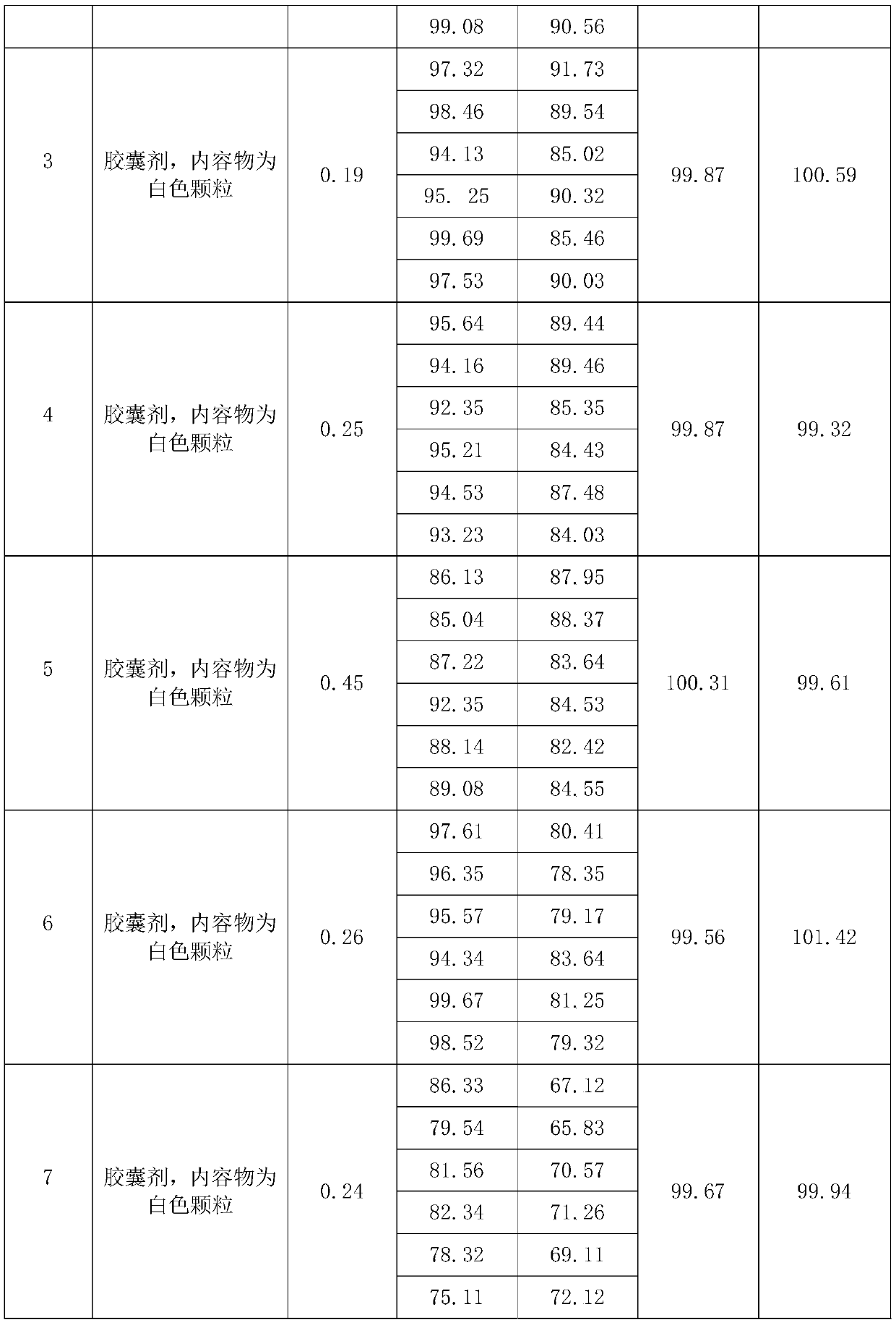
Embodiment 2
[0036] Embodiment 2 (500,000 grains)
[0037] A preparation method of valsartan hydrochlorothiazide capsules, comprising the following steps:
[0038] S1. Weigh valsartan, hydrochlorothiazide, disintegrant, cosolvent, filler, lubricant, and binder according to the proportion, wherein the disintegrator is sodium carboxymethyl starch, and the filler is microcrystalline Cellulose and cornstarch, described solubilizer is polyethylene glycol 6000, described lubricant is magnesium stearate, described binding agent is povidone K30;
[0039] S2, preparing a binder solution, adding the binder weighed in proportion to purified water to prepare a binder solution with a weight ratio of 5-10%, stirring evenly for later use;
[0040] S3. Mix and pulverize valsartan, 50% disintegrant, and filler through an 80-mesh sieve to obtain a mixed material, mix and pulverize hydrochlorothiazide and a cosolvent through an 80-mesh sieve to obtain hydrochlorothiazide powder;
[0041] S4. Add the mixed ...
Embodiment 3
[0048] Embodiment 3 (500,000 grains)
[0049] A preparation method of valsartan hydrochlorothiazide capsules, comprising the following steps:
[0050] S1. Weigh valsartan, hydrochlorothiazide, disintegrant, cosolvent, filler, lubricant, and binder according to the proportion, wherein the disintegrator is sodium carboxymethyl starch, and the filler is microcrystalline Cellulose and cornstarch, described solubilizer are meglumine, described lubricant is magnesium stearate, described binding agent is povidone K30;
[0051] S2, preparing a binder solution, adding the binder weighed in proportion to purified water to prepare a binder solution with a weight ratio of 5-10%, stirring evenly for later use;
[0052] S3. Mix and pulverize valsartan, 50% disintegrant, and filler through an 80-mesh sieve to obtain a mixed material, mix and pulverize hydrochlorothiazide and a cosolvent through an 80-mesh sieve to obtain hydrochlorothiazide powder;
[0053] S4. Add the mixed material obtai...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com