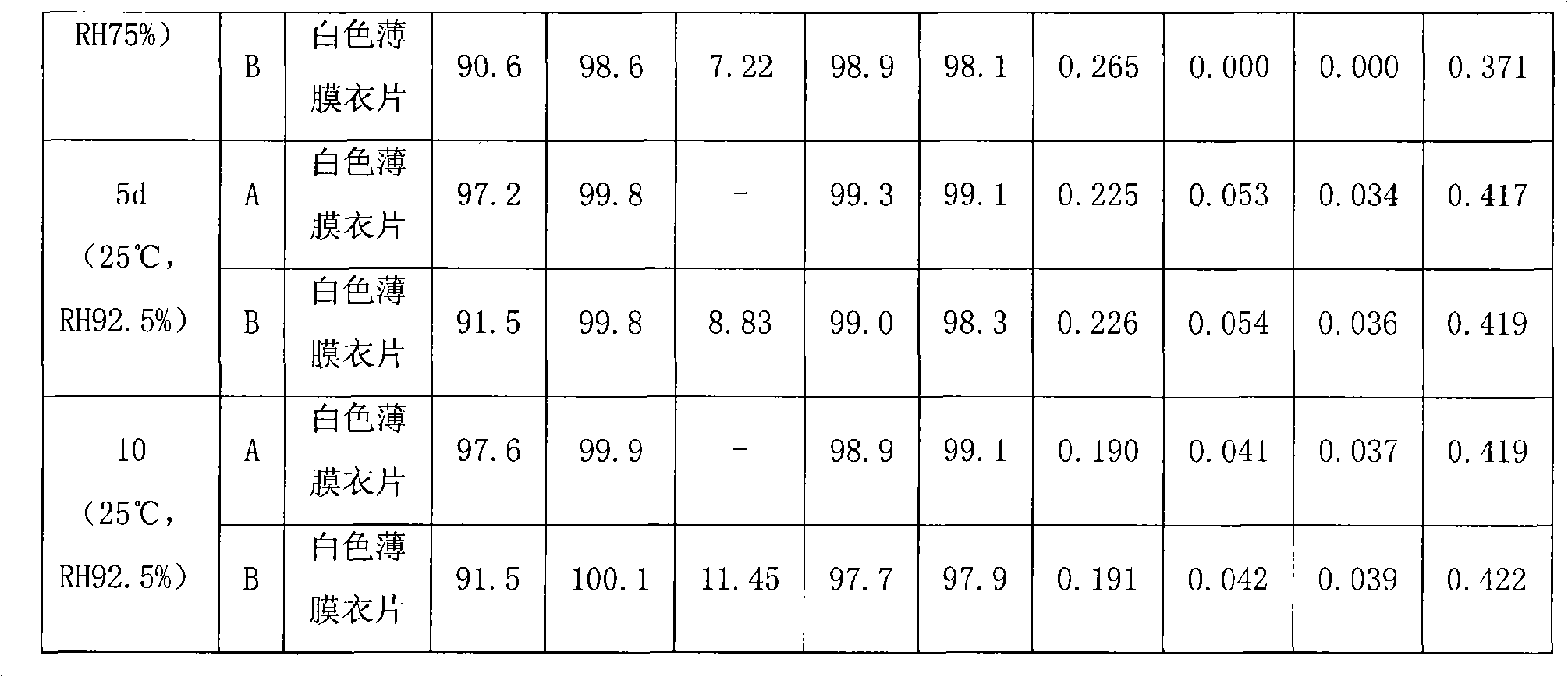
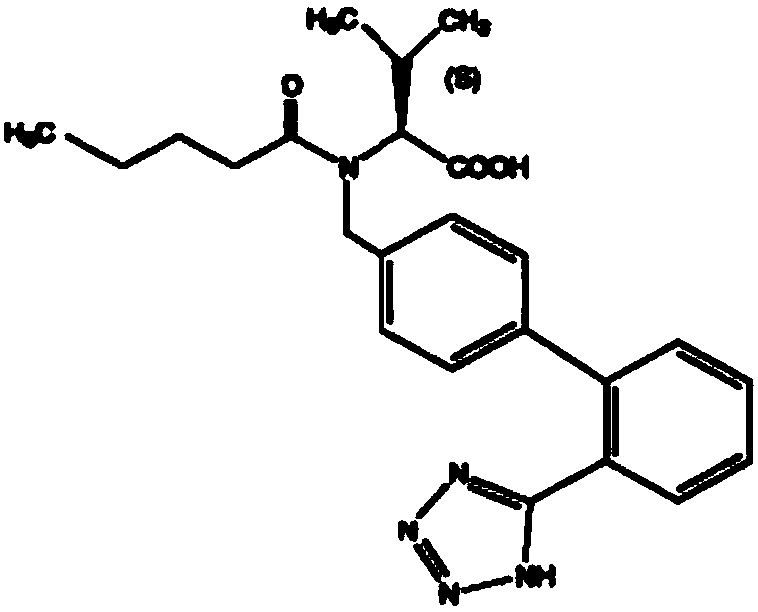
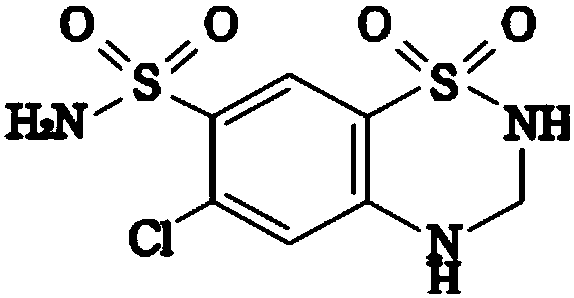
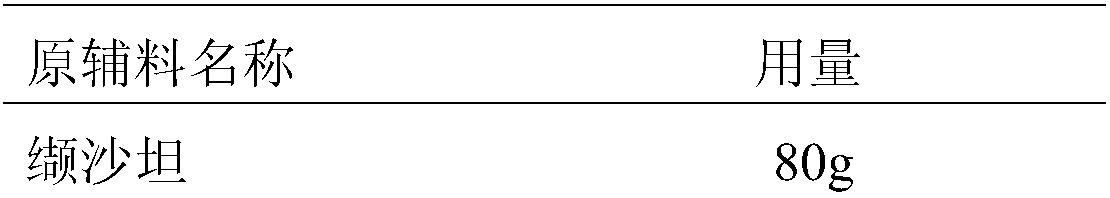
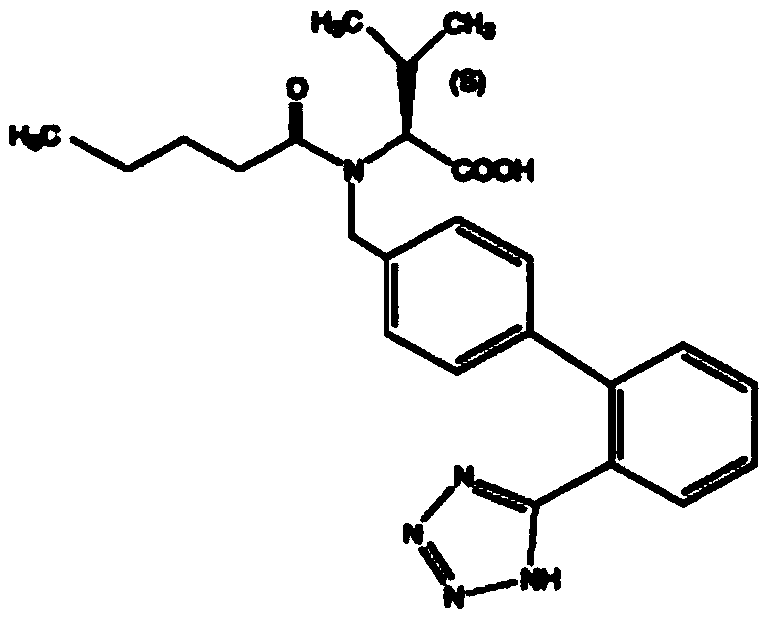
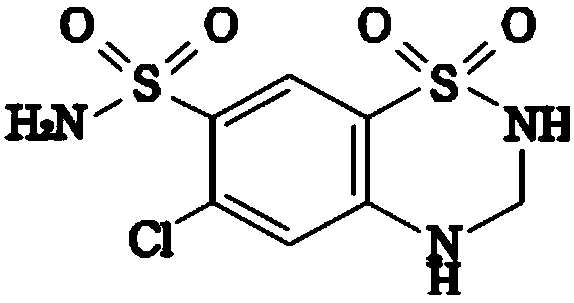
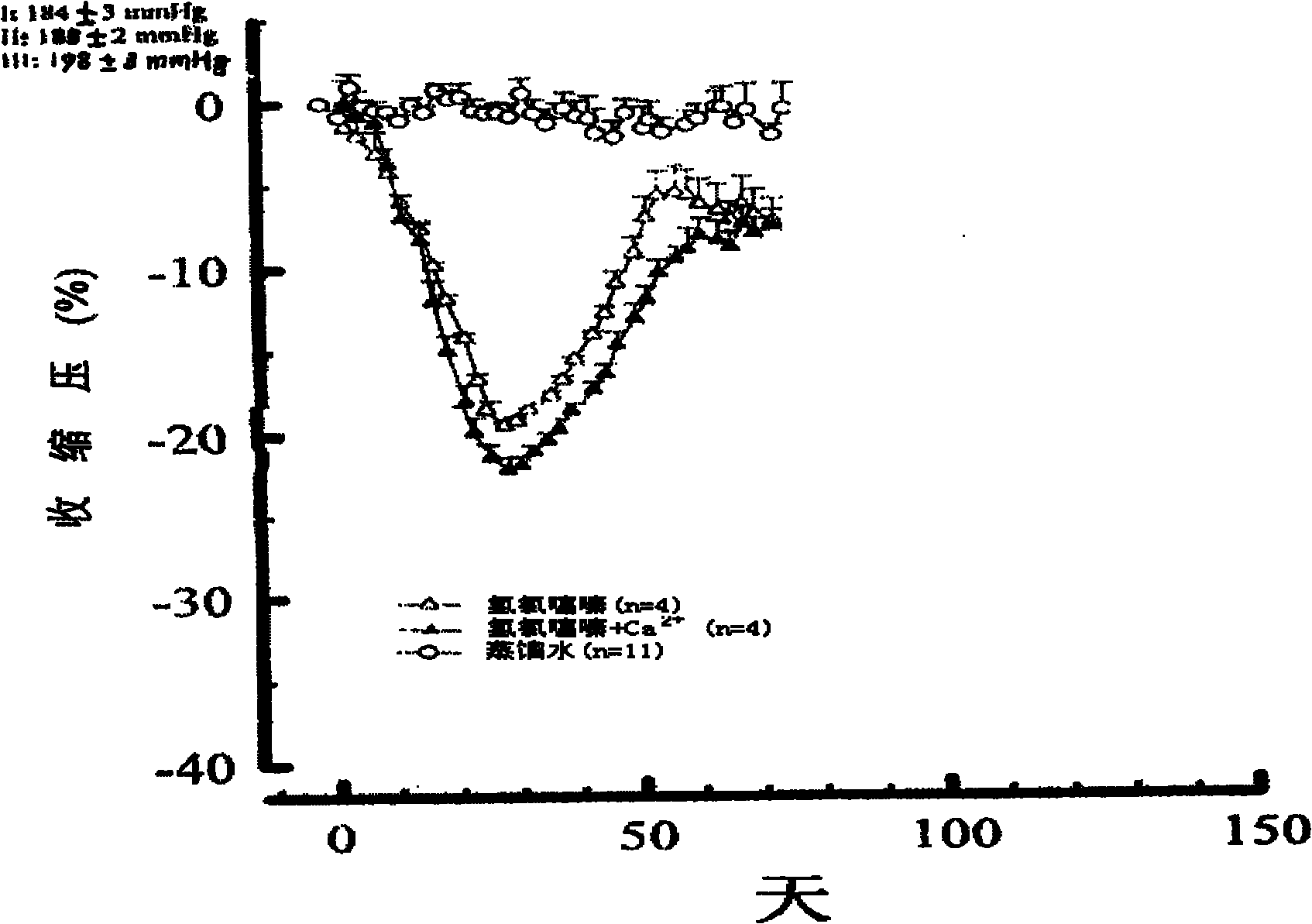
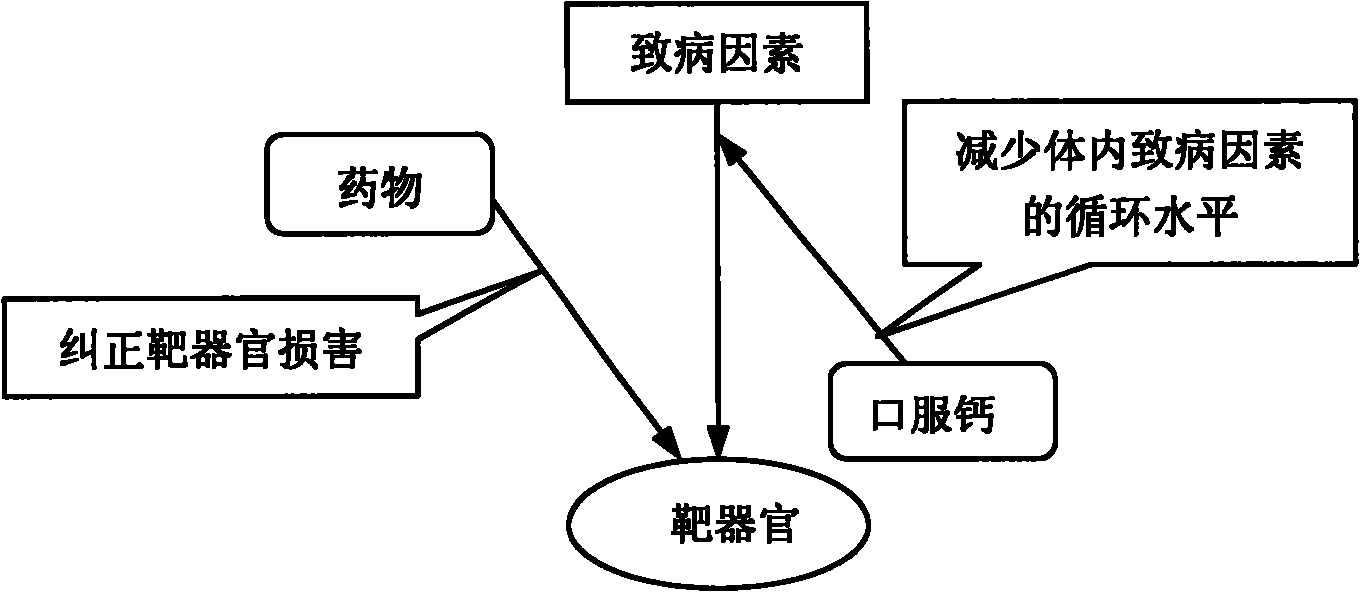
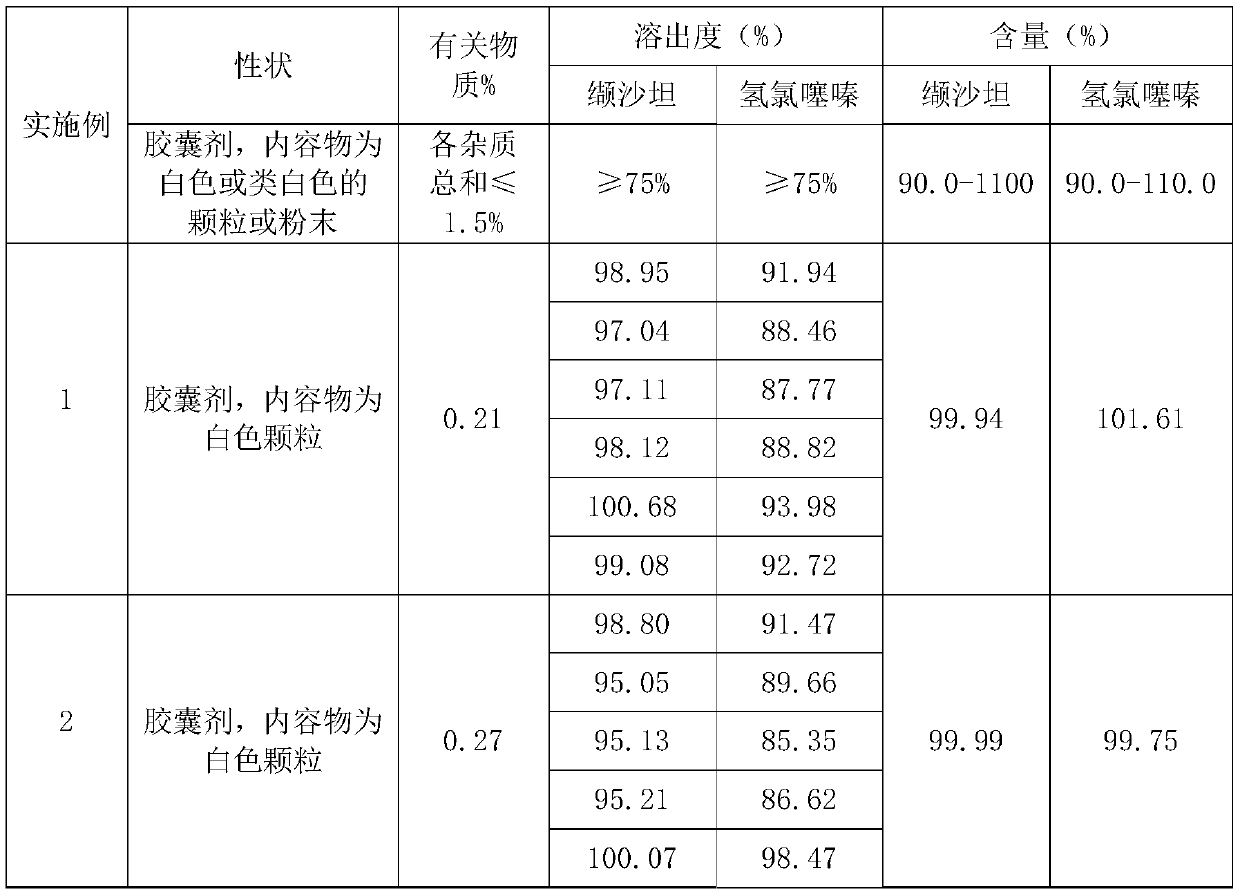
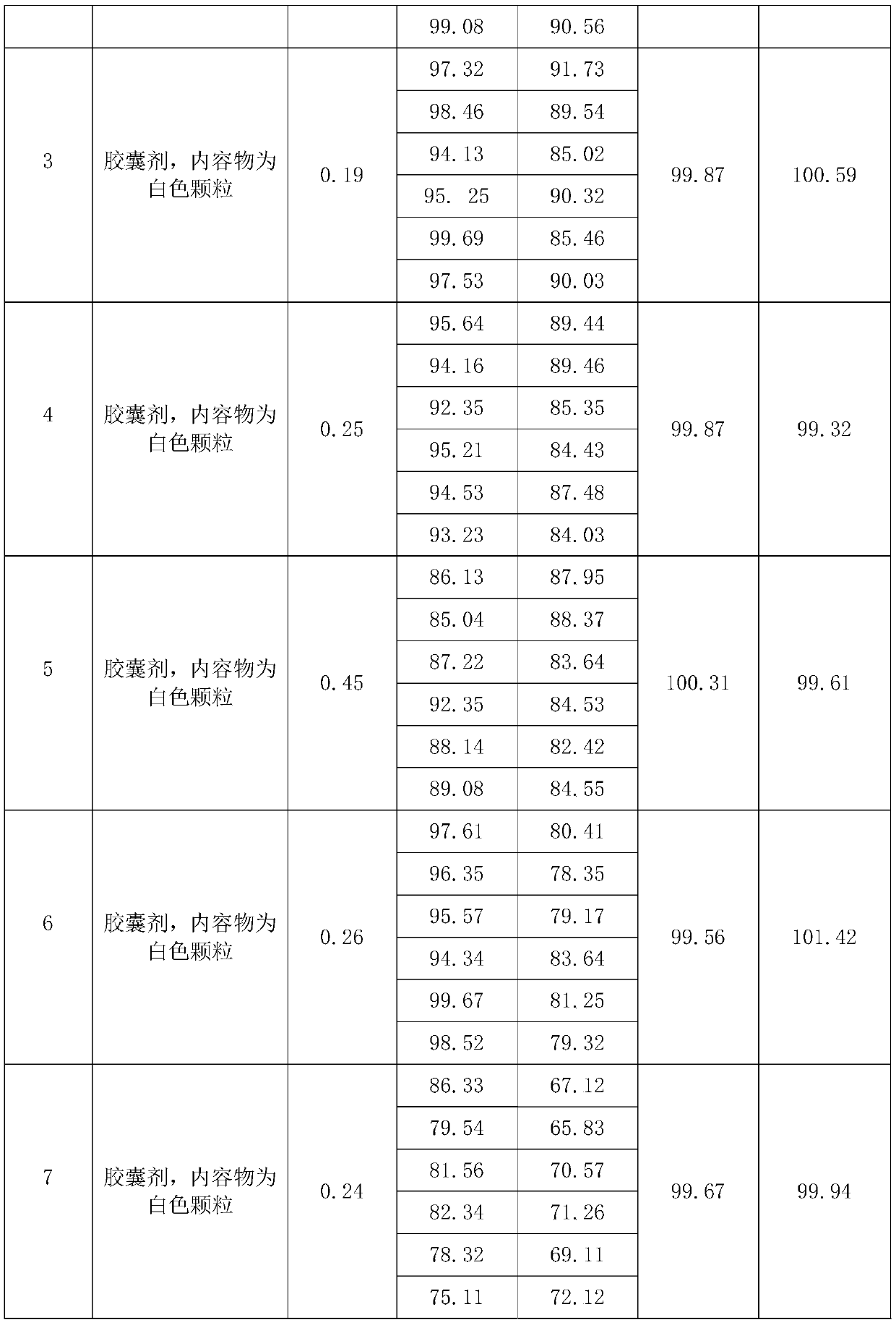
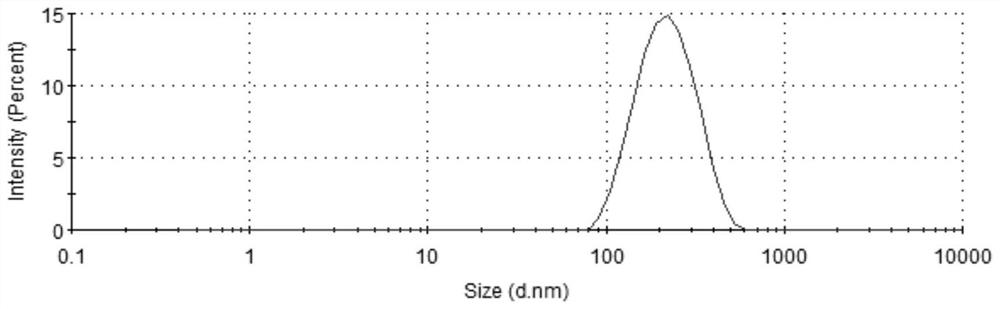
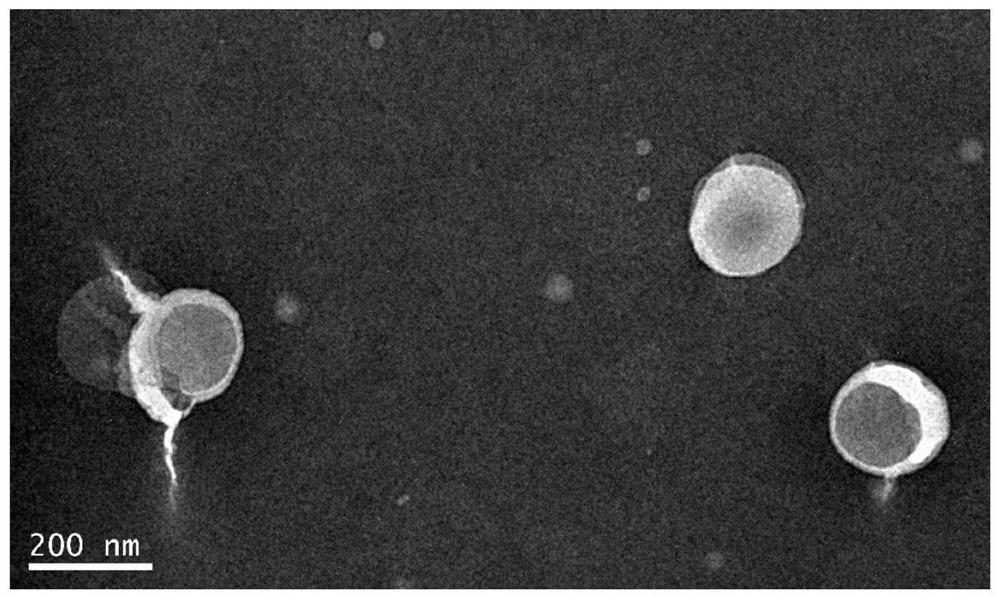
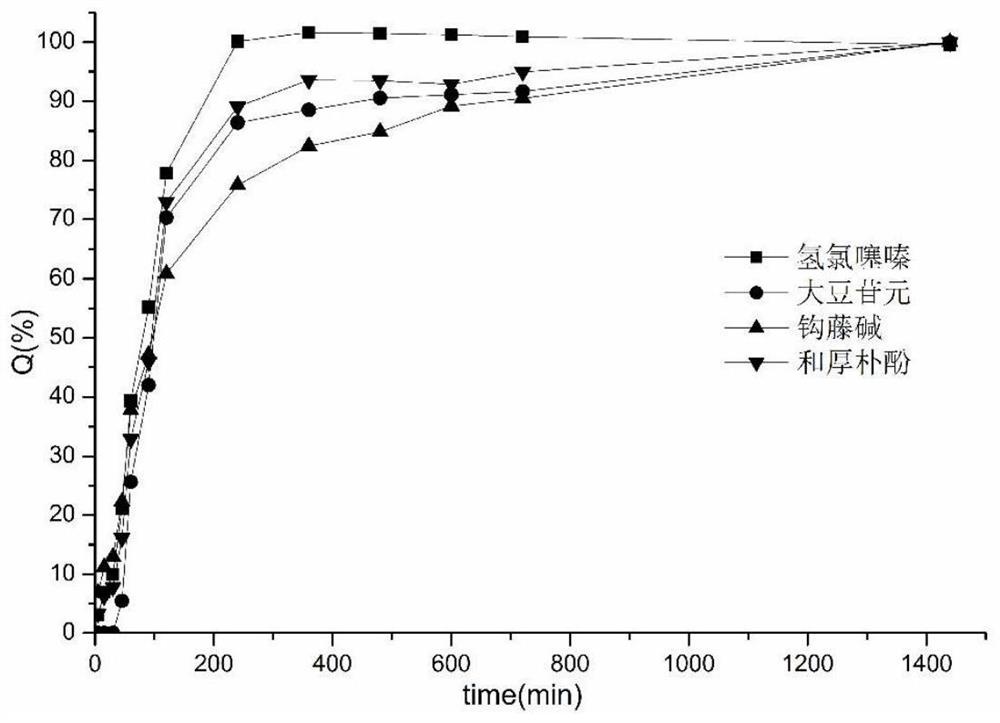
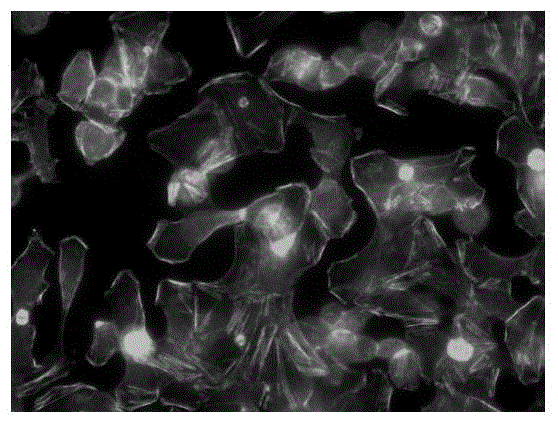
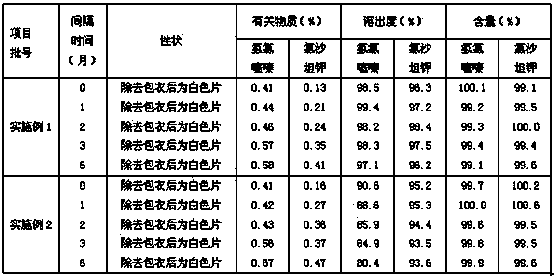
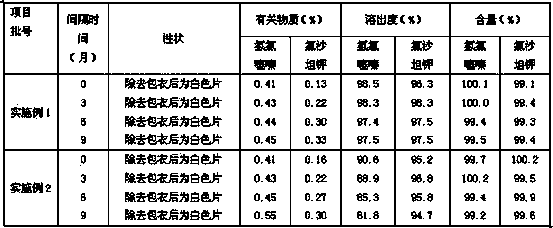
Patents
Literature
47 results about "Chlorothiazide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
This medication is used to treat high blood pressure.
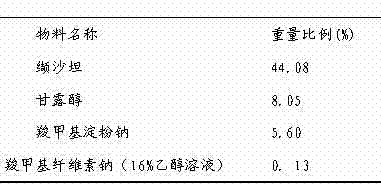
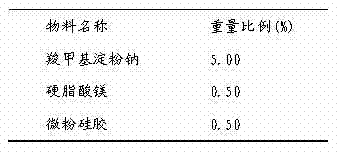
Medicinal composition and preparation method thereof
InactiveCN102526061AReduce hydrophobicityReduce viscosityPharmaceutical non-active ingredientsCardiovascular disorderValsartanHydrochlorothiazide
The invention discloses a medicinal composition. The medicinal composition consists of the following ingredients in percentage by weight: 1 to 5 percent of amlodipine and pharmaceutically acceptable salt thereof, 20 to 50 percent of valsartan, 1 to 10 percent of hydrochlorothiazide, 20 to 50 percent of filling agent, 8 to 24 percent of disintegrating agent, 0.01 to 0.06 percent of adhesive 1, 0.01 to 0.2 percent of adhesive 2, 0.3 to 2 percent of flow aid and 0.5 to 2.5 percent of lubricating agent. The medicinal composition has the advantages of simple preparation process, low equipment requirement, simplicity in operation and small dust pollution, guarantees the quality of medicines and contributes to industrialized production.
Owner:BEIJING D VENTUREPHARM TECH DEV
Composite tablet of valsartan and hydrochlorothiazide and preparation method thereof
ActiveCN102631357AActive ingredients have strong absorption capacityGood treatment effectMetabolism disorderDigestive systemCelluloseValsartan
The invention provides a composite tablet of valsartan and hydrochlorothiazide, and the composite tablet is prepared from valsartan, hydrochlorothiazide, phosphatidylcholine, magnesium carbonate, magnesium carbonate, starch, hydroxy propyl cellulose, powered sugar and silica, wherein the valsartan, the hydrochlorothiazide and the phosphatidylcholine are active ingredients. According to the composite tablet provided by the invention, the absorption capability of the active ingredients is stronger, and the therapeutic effect to the primary hypertension is better; and moreover, the composite tablet can be used for protecting the liver, and meanwhile has therapeutic effects to the left ventricular hypertrophy caused by hypertension.
Owner:CHONGQING CONQUER PHARML
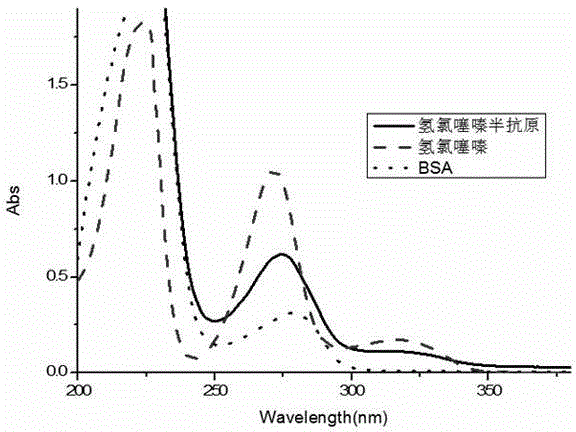
Hydrochlorothiazide semi-antigen and complete antigen as well as preparation method thereof
InactiveCN104447619AStrong specificityHigh sensitivityOvalbuminSerum albuminSerum igeHydrochlorothiazide
The invention discloses hydrochlorothiazide semi-antigen and complete antigen as well as a preparation method thereof, and belongs to the technical field of biochemical industry. The hydrochlorothiazide semi-antigen is obtained by dissolving hydrochlorothiazide in methanol, adding potassium carbonate, then reacting with 6-bromine ethyl caproate, and performing extractive separation. The hydrochlorothiazide complete antigen is obtained by coupling carboxyl groups on the hydrochlorothiazide semi-antigen with amino groups on carrier protein. Experimental results show that the antiserum titer obtained by using the hydrochlorothiazide complete antigen disclosed by the invention to immunize animals can reach 81000, the limit of detection is 0.01ng / mL, and the half inhibiting concentration is 0.1ng / mL. Generated antibodies are high in specificity and sensitivity. The antigens or the antibodies disclosed by the invention have a wide application prospect.
Owner:JIANGNAN UNIV
Losartan potassium and hydrochlorothiazide tablets and preparation method thereof
ActiveCN101327195AHigh dissolution rateLow hygroscopicityPharmaceutical non-active ingredientsPill deliveryChemistryMagnesium stearate
The invention relates to a losartan potassium hydrochlorothiazide tablet and a preparation method thereof. The losartan potassium hydrochlorothiazide tablet comprises a core and a film coat layer and is characterized in that the core is composed of the losartan potassium and the hydrochlorothiazide which serve as the medicinal active components, and a medicinally available accessory. The medicinally available accessory is microcrystalline cellulose, pregelatinized starch, lactose monohydrate, polyvinylpyrrolidone K30 and magnesium stearate. The preparation method of the losartan potassium hydrochlorothiazide tablet is as the following: the hydrochlorothiazide is mixed with the easily-dissolvable losartan potassium and lactose monohydrate, and then the mixture is made into grains; the hydrochlorothiazide is dispersed in the easily-dissolvable losartan potassium and lactose monohydrate, and then mixed with the microcrystalline cellulose, the pregelatinized starch and magnesium stearate; then tablets are made and coated. The tablet hydrochlorothiazide prepared according to the method of the invention dissolves out well and has no moisture absorption under high moisture conditions.
Owner:HAINAN JINRUI PHARMA
Valsartan/hydrochlorothiazide tablet and preparation method thereof
ActiveCN108567759AHigh dissolution rateGood chemical stabilityPharmaceutical non-active ingredientsCoatingsMedicineDissolution
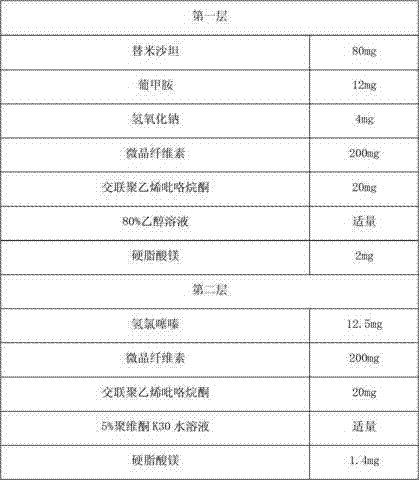
The invention provides a valsartan / hydrochlorothiazide tablet and a preparation method thereof. The valsartan / hydrochlorothiazide tablet is prepared from the following raw material substances in partsby mass: 80 parts of valsartan, 12.5 parts of hydrochlorothiazide, 20 to 28 parts of microcrystalline cellulose PH102, 3 to 10 parts of copovidone S630, 1.0 to 2.0 parts of colloidal silicon dioxide,18 to 22 parts of polyvinylpolypyrrolidone XL, 3.50 to 5.00 parts of magnesium stearate and 3 to 7.5 parts of film coating premixing agents (gastric dissolution type), wherein the mass part ratio ofmicrocrystalline cellulose to copovidone S630 is (3 to 4):1. The copovidone S630 is used for replacing partial microcrystalline cellulose PH102; the dissolution rate of valsartan and the hydrochlorothiazide is increased; in addition, copovidone S630 is added into the coating solution, so that the water sensitivity of the valsartan / hydrochlorothiazide tablet is improved, so that the chemical stability of valsartan is enhanced.
Owner:BEIJING BAIAO PHARMA
Compound composition for treatment of high blood pressure and preparation method thereof
InactiveCN104510738ASolve liquidity problemsSolve problems such as sticking and punchingPharmaceutical non-active ingredientsHeterocyclic compound active ingredientsHypertension medicationsValsartan
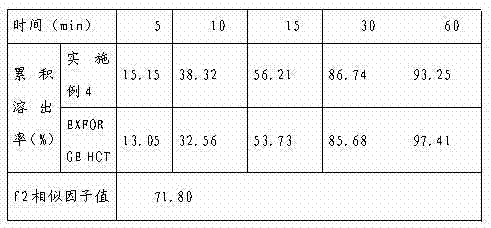
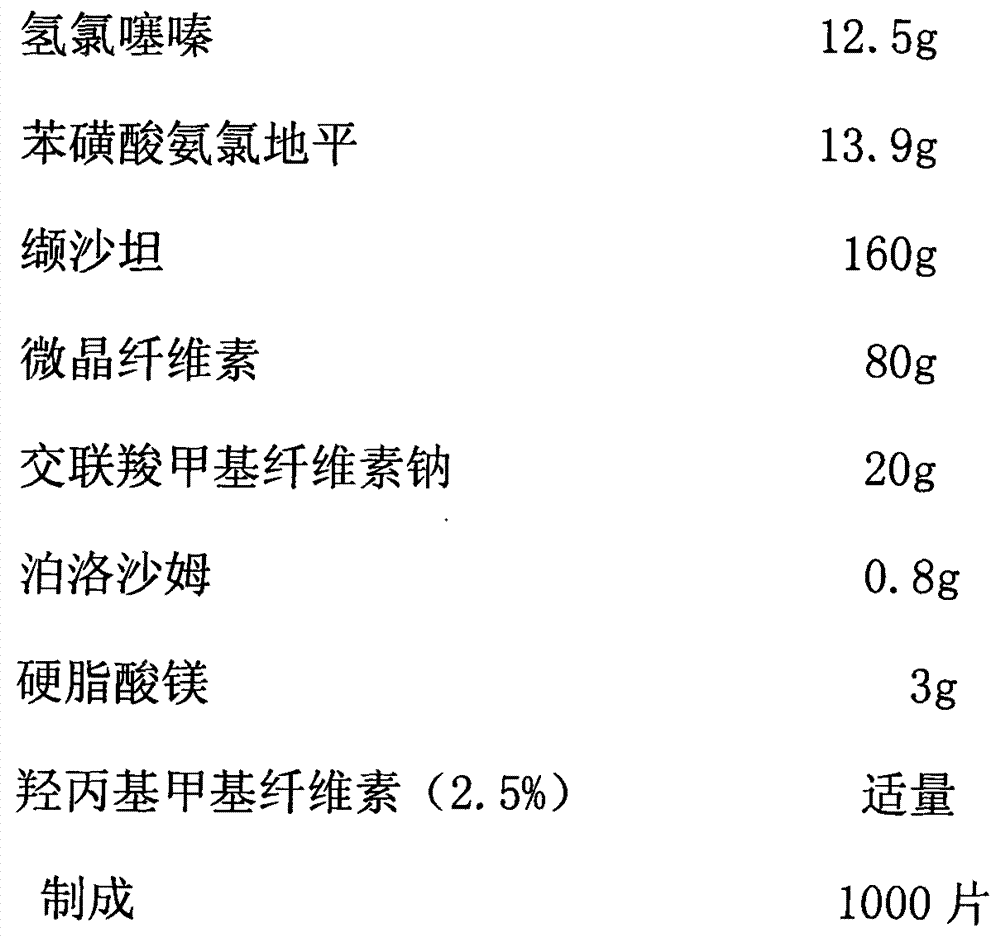
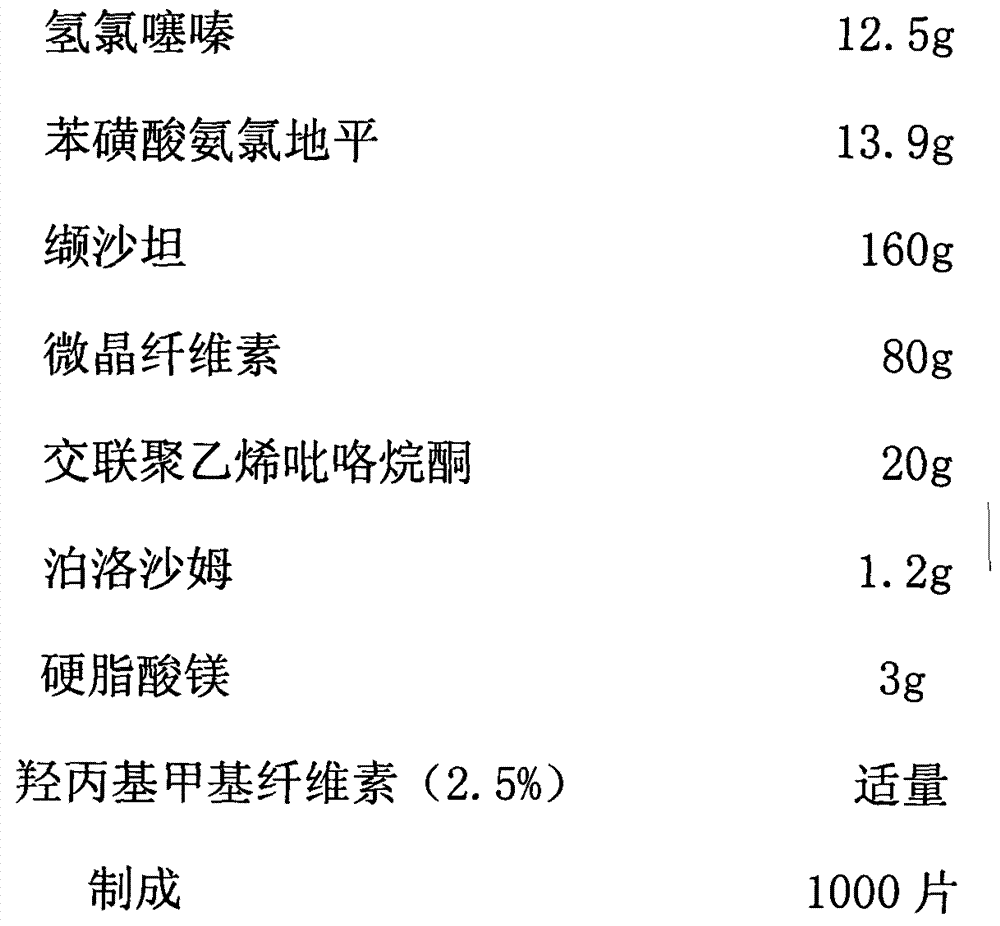
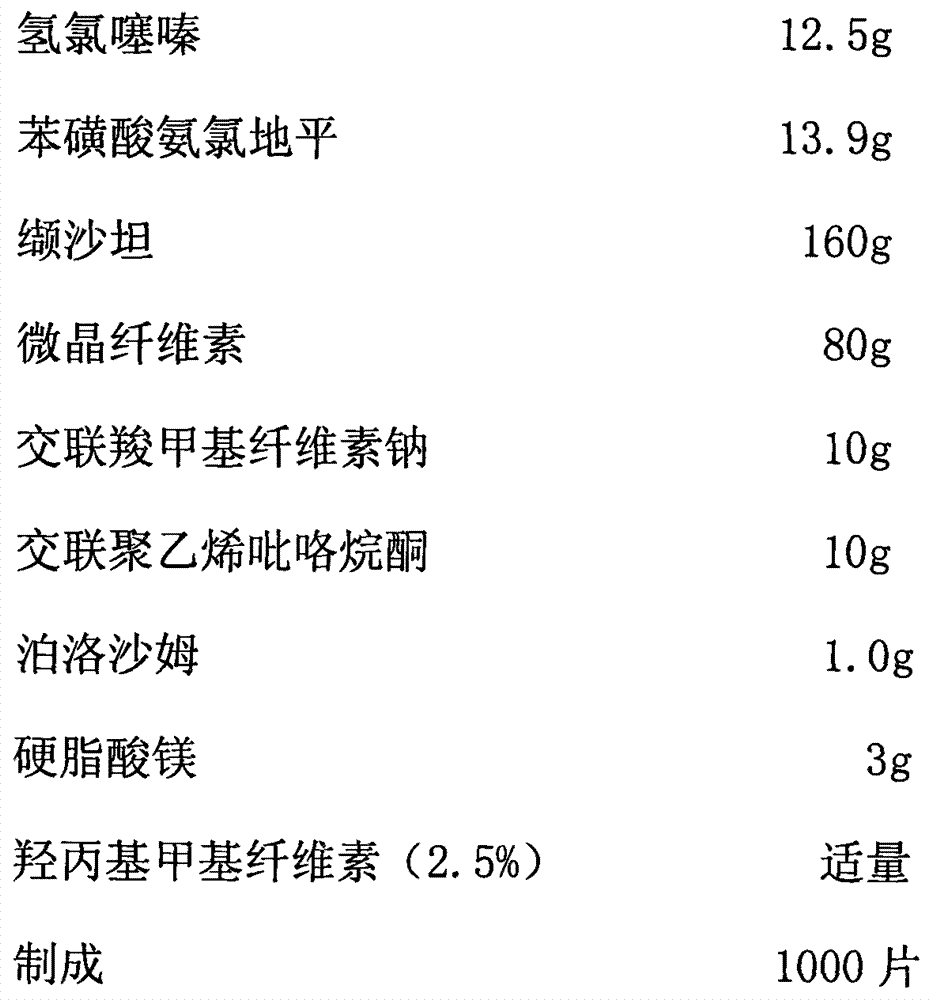
The invention discloses a compound oral preparation and a preparation method thereof. The compound oral preparation is used for treatment of high blood pressure and has significant effect. The preparation includes three anti-hypertensive drugs hydrochlorothiazide, amlodipine besylate, valsartan and pharmaceutical excipients. The three components all belong to hydrophobic drugs, so that the dissolution rate is one of the key indicators measuring the intrinsic quality of the preparation. A specific ratio of the raw materials to the excipients is adopted by the invention, especially a polyoxyethylene-polyoxypropylene copolymer is used, for instance poloxamer serves as a solubilizing agent, so that compared with the anti-hypertensive compound preparation EXFORGE HCT produced by American Novartis pharmaceutical company, the compound preparation has good similarity in terms of dissolution behavior. According to the invention, wet granulation or dry granulation is employed to effectively solve the problems of low bulk density, poor liquidity, strong moisture absorption, and sticking and picking of Valsartan. The preparation method of the compound preparation is simple and practical, and the product has stable quality, and good dissolution rate and content uniformity of each effective component, thus being suitable for large-scale industrial production.
Owner:TIANJIN CHANGSHOUYUAN HEALTH TECH CO LTD
Compound preparation containing losartan potassium and hydrochlorothiazide and preparation method for compound preparation
InactiveCN102526063ARapid dissolutionImprove stabilityOrganic active ingredientsPharmaceutical delivery mechanismAdditive ingredientLow-substituted hydroxypropylcellulose
The invention relates to a compound preparation containing losartan potassium and hydrochlorothiazide and a preparation method for the compound preparation. The compound preparation containing the losartan potassium and the hydrochlorothiazide comprises a tablet core and a film coating, wherein the tablet core comprises active pharmaceutical ingredients of the losartan potassium and the hydrochlorothiazide, and minor pharmaceutical ingredients such as microcrystalline cellulose, lactose, low substituted hydroxyprepyl cellulose and magnesium stearate. The prepared compound preparation has high dissolubility, is simple in preparation process and can be easily produced in large scale; and moreover, the degradation speed and degree of the hydrochlorothiazide can be remarkably lowered, and impurities 4-amino-6-chloro-1,3-benzenedisulfonamide (DSA) and chlorothiazide can be prevented from increasing.
Owner:CHINA PHARM UNIV
Compound telmisartan hydrochlorothiazide pharmaceutical composition and preparation method thereof
InactiveCN102846624AReasonable prescriptionImprove product qualityOrganic active ingredientsDrageesCoated tabletsHydrochlorothiazide
The invention discloses a compound telmisartan hydrochlorothiazide pharmaceutical composition, which is a tablet. For the tablet, hydrochlorothiazide and telmisartan are prepared into a coated tablet so as to make the telmisartan and the hydrochlorothiazide in the telmisartan hydrochlorothiazide pharmaceutical composition respectively released. The composition has a controlled release effect and improves the bioavailability. The employment of a coated tablet preparation process can make the two medicines effectively released successively. The pharmaceutical composition has a controlled release effect, maintains a long-term blood concentration, has a more stable blood pressure reduction effect, and reduces adverse reactions.
Owner:庞晓斌
Preparation method for compound losartan potassium-hydrochlorothiazide pharmaceutical composition
ActiveCN102475707AMature technologyEasy to operateOrganic active ingredientsPill deliveryCelluloseCross-link
The invention relates to a preparation method for a losartan potassium-hydrochlorothiazide tablet. The losartan potassium-hydrochlorothiazide tablet is characterized in that: after losartan potassium and starch are mixed, 10% of starch slurry is used to prepare particles with a suitable hardness; hydrochlorothiazide, cross linked sodium carboxymethyl cellulose and lactose are mixed, and particles with a proper hardness are prepared from an obtained mixture by using 5% of a polyvinylpyrrolidone K30 solution; the two kinds of particles and magnesium stearate are uniformly mixed and then are subjected to tabletting. According to the invention, a low dissolution rate caused by interaction among drugs in primary granulation is avoided, and an in vitro dissolution rate can be improved greatly, thereby enhancing bioavailability.
Owner:TIANJIN HANKANG PHARMA BIOTECH
Fingerprint spectrum detection method of Zhenju anti-hypertension tablets and application thereof
ActiveCN103499653AQuick and easy to manufactureEfficient preparationComponent separationChlorogenic acidAnti hypertension
The invention provides a fingerprint spectrum detection method of Zhenju anti-hypertension tablets and application of the method. The fingerprint spectrum detection method of the Zhenju anti-hypertension tablets comprises the steps of preparation of a test solution, namely evenly mixing a ground test sample and a methyl alcohol aqueous solution extract solvent with the volume percent of more than 30%, and then carrying out ultrasonic treatment for more than 30min to obtain the test solution after solid-liquid separation; measurement of the test solution, namely under appropriate conditions, measuring or obtaining the fingerprint spectrum of the test solution by high performance liquid chromatography. The invention further provides the application of the fingerprint spectrum detection method to the quality detection of the Zhenju anti-hypertension tablets or the content measurement of one or more of chlorogenic acid, galuteolin, linarin, hydrochlorothiazide, rutin and the like in the Zhenju anti-hypertension tablets. The method not only is suitable for measuring the finished product of the Zhenju antihypertension tablets, but also can be used for guiding the production process control or the technology improvement.
Owner:SHANGHAI LEIYUNSHANG PHARMA
Blood pressure lowering dripping pills with chryanthemum flower and pearl and its preparation process
InactiveCN1698797AFaster dissolution and absorptionImprove bioavailabilityPill deliveryCardiovascular disorderOral drug preparationClonidine Hydrochloride
Disclosed is a dripping pill for treating hypertension. The objective of the invention is to provide a medicinal composition having the advantages of high biological availability, quick-speed medicine release, quick-speed effect, higher medicinal content, easy administration, low price, and causing no pollution during production. The drop pill is prepared from powdered wild chrysanthemum flower, pearl powder, clonidine hydrochloride, hydrochlorothiazidum, globularicitrin as raw material, and medicinal carrying agent as the base material.
Owner:广西宝瑞坦广明制药有限公司
Brand new levamlodipine and hydrochlorothiazide medicinal composition and preparation method thereof
ActiveCN102327271AOrganic active ingredientsOrganic chemistryCarboxymethyl starchHydrochlorothiazide
The invention discloses a brand new levamlodipine and hydrochlorothiazide medicinal composition and a preparation method thereof. The medicinal composition is an oral preparation which is prepared by adding pharmaceutically acceptable auxiliary materials into hydrochlorothiazide crystals and levamlodipine; and the oral preparation comprises but is not limited to tablets or capsules. The composition comprises the following components in part by weight: 2.5 to 5 parts of levamlodipine, 6.25 to 12.5 parts of hydrochlorothiazide crystal, 10 to 45 parts of pregelatinized starch, 20 to 25 parts of carboxymethyl starch, 15 to 35 parts of microcrystalline cellulose PH102, 10 to 45 parts of hydroxy propyl cellulose, and 0.5 to 1 part of magnesium stearate. The medicinal composition is reasonable in a prescription, stable and reliable in quality and good in disintegration time and high in dissolution rate; and a process for directly tabletting by using powder is adopted, the process is simple, the production cycle is short, production cost is low, and industrial production is easy to realize.
Owner:HAINAN JINRUI PHARMA
Fosinopril sodium and hydrochlorothiazide pharmaceutical composition and preparation method thereof
ActiveCN102327277AOrganic active ingredientsOrganic chemistryCarboxymethyl starchHydrochlorothiazide
The invention discloses a fosinopril sodium and hydrochlorothiazide pharmaceutical composition and a preparation method thereof. The pharmaceutical composition is a tablet or capsule prepared from hydrochlorothiazide crystal, fosinopril sodium and pharmaceutically acceptable auxiliary materials. The composition comprises the following components in parts by weight: 5-45 parts of fosinopril sodium, 5-30 parts of hydrochlorothiazide crystal, 10-50 parts of pregelatinized starch, 20-25 parts of sodium carboxymethyl starch, 15-35 parts of microcrystalline cellulose PH102, 10-45 parts of hydroxypropyl cellulose and 0.5-1 part of magnesium stearate. The pharmaceutical composition has the advantages of reasonable prescription, stable and reliable quality, and better disintegration time limit and dissolution rate. In the invention, a powder direct tabletting process is adopted, thus the preparation method has the advantages of simple process, short production period and low production cost and can easily realize industrial production.
Owner:HAINAN JINRUI PHARMA
Composition containing clerodendranthus spicatus aqueous extract
InactiveCN101693050AFully extractedOrganic active ingredientsUrinary disorderHydrochlorothiazideAdditive ingredient
The invention discloses a composition containing a clerodendranthus spicatus aqueous extract, which comprises the clerodendranthus spicatus aqueous extract and hydrochlorothiazide, wherein the weight ratio of the clerodendranthus spicatus aqueous extract and the hydrochlorothiazide which have the weight being equivalent to 1 gram of primary medicine is 1:0.005-0.01. Compared with the prior art, the invention has the advantages that the water-soluble beneficial components packaged in cellulose can be sufficiently extracted by using composite cellulase to dissolve the cellulose in clerodendranthus spicatus stalks in the extracting process of the clerodendranthus spicatus aqueous extract and simultaneously the litholytic and diuretic functions of the clerodendranthus spicatus aqueous extract are enhanced by using the hydrochlorothiazide.
Owner:芜湖梁氏新材料有限公司
Hydrochlorothiazide orally disintegrating tablets and preparation method thereof
InactiveCN103830191AFast dissolution rateQuick effectOrganic active ingredientsPill deliveryHydrochlorothiazideOrally disintegrating tablet
The invention provides hydrochlorothiazide orally disintegrating tablets and a preparation method thereof. A patient does not need to drink water for taking the orally disintegrating tablets, thereby reducing the burden on the patient; moreover, by adopting a tabletting pretreatment process on the orally disintegrating tablets, the problems that a large quantity of accessories insoluble in water are adopted and insoluble substances are left after the disintegration are successfully solved.
Owner:QINGDAO BAICAOHUI INST OF CHINESE HERBAL MEDICINE
Dough sculpture for preventing from cracking and getting mildew
The invention mainly relates to the technical field of dough sculpture material processing, and discloses a dough sculpture for preventing from cracking and getting mildew. The dough sculpture is prepared from the following raw materials: 400 to 500 parts of low-gluten flour, 140 to 160 parts of konjac starch, 16 to 18 parts of table salt, 7 to 9 parts of methylcellulose, 7 to 9 parts of microcrystalline cellulose, 5 to 7 parts of ginger root extract, 2 to 3 parts of nano-zinc oxide, 0.23 to 0.25 part of chlorothiazide, and 300 to 400 parts of water. The dough sculpture for preventing from cracking and getting mildew provided by the invention is smooth in surface, bright in color, free of getting mildew or cracking, and capable of being stored for 14 to 16 years under the indoor opened environment so as to be stored for a long time; the nano-zinc oxide is added into the low-gluten flour, and proofing is carried out after low-speed stirring, so that the water content of a dough is uniform, and the texture is fine and soft; and mucedin is decomposed, so that the elasticity is reduced, and the dough sculpture is easy to form.
Owner:界首市翰墨文化艺术传媒有限公司
A kind of valsartan hydrochlorothiazide tablet and preparation method thereof
ActiveCN108567759BHigh dissolution rateGood chemical stabilityPharmaceutical non-active ingredientsCoatingsMagnesium stearateStearic acid
The invention provides a valsartan / hydrochlorothiazide tablet and a preparation method thereof. The valsartan / hydrochlorothiazide tablet is prepared from the following raw material substances in partsby mass: 80 parts of valsartan, 12.5 parts of hydrochlorothiazide, 20 to 28 parts of microcrystalline cellulose PH102, 3 to 10 parts of copovidone S630, 1.0 to 2.0 parts of colloidal silicon dioxide,18 to 22 parts of polyvinylpolypyrrolidone XL, 3.50 to 5.00 parts of magnesium stearate and 3 to 7.5 parts of film coating premixing agents (gastric dissolution type), wherein the mass part ratio ofmicrocrystalline cellulose to copovidone S630 is (3 to 4):1. The copovidone S630 is used for replacing partial microcrystalline cellulose PH102; the dissolution rate of valsartan and the hydrochlorothiazide is increased; in addition, copovidone S630 is added into the coating solution, so that the water sensitivity of the valsartan / hydrochlorothiazide tablet is improved, so that the chemical stability of valsartan is enhanced.
Owner:BEIJING BAIAO PHARMA
Composition of medication for treating high blood pressure
InactiveCN1695738BLower blood pressureOrganic active ingredientsCardiovascular disorderBumetanideHigh pressure
Owner:苏金平
A kind of preparation method of valsartan hydrochlorothiazide capsule
ActiveCN106983752BHigh dissolution rateSimple processCapsule deliveryHeterocyclic compound active ingredientsBiochemistryValsartan/hydrochlorothiazide
Owner:CHONGQING CONQUER PHARML
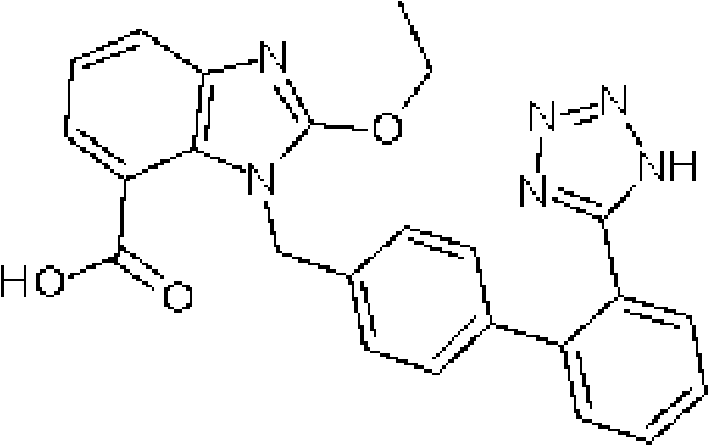
Candesartan cilexetil and hydrochlorothiazide co-amorphous substance and preparation method thereof
ActiveCN111888361AImprove solubilityIncrease dissolution ratePowder deliveryOrganic active ingredientsHydrochlorothiazideActive ingredient
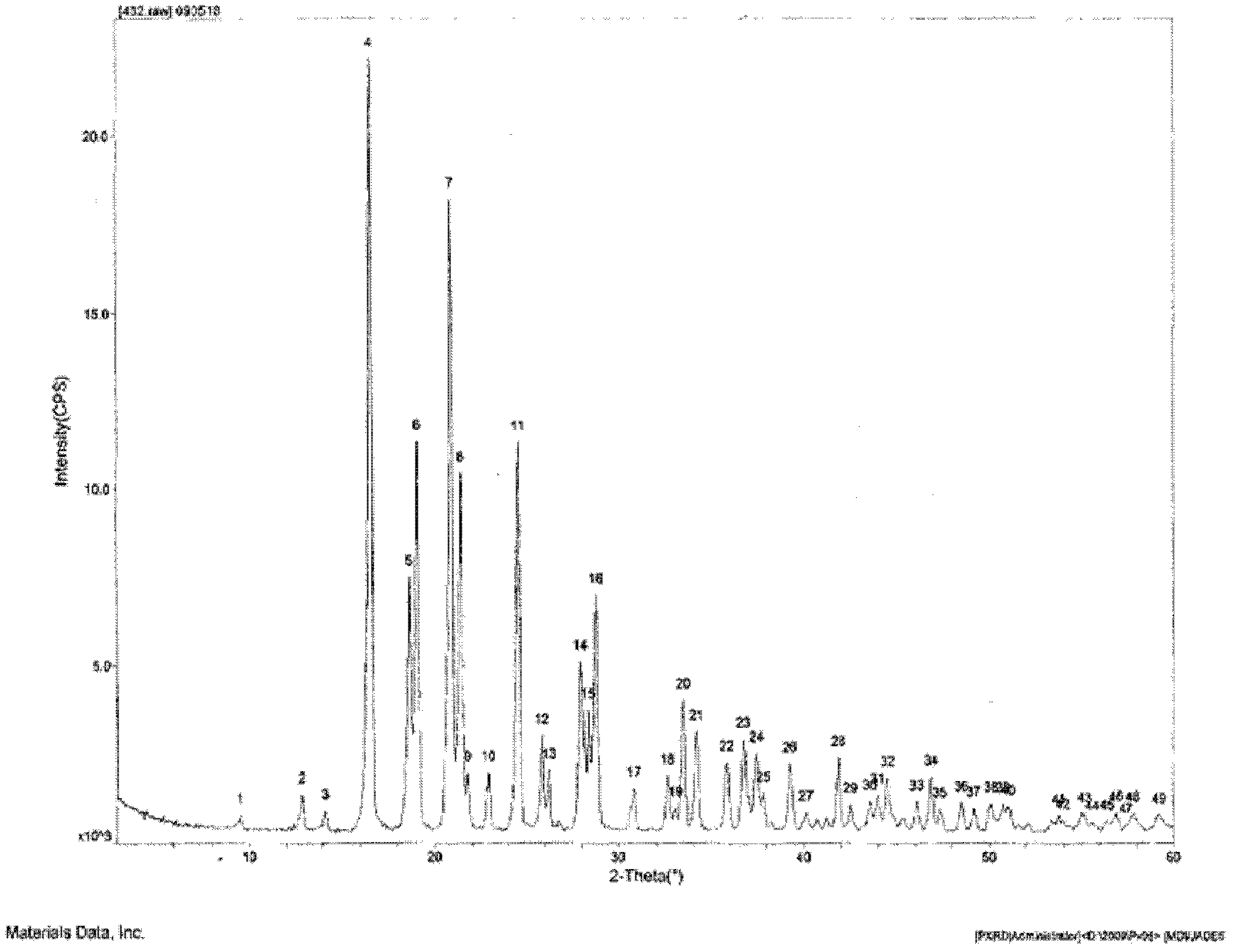
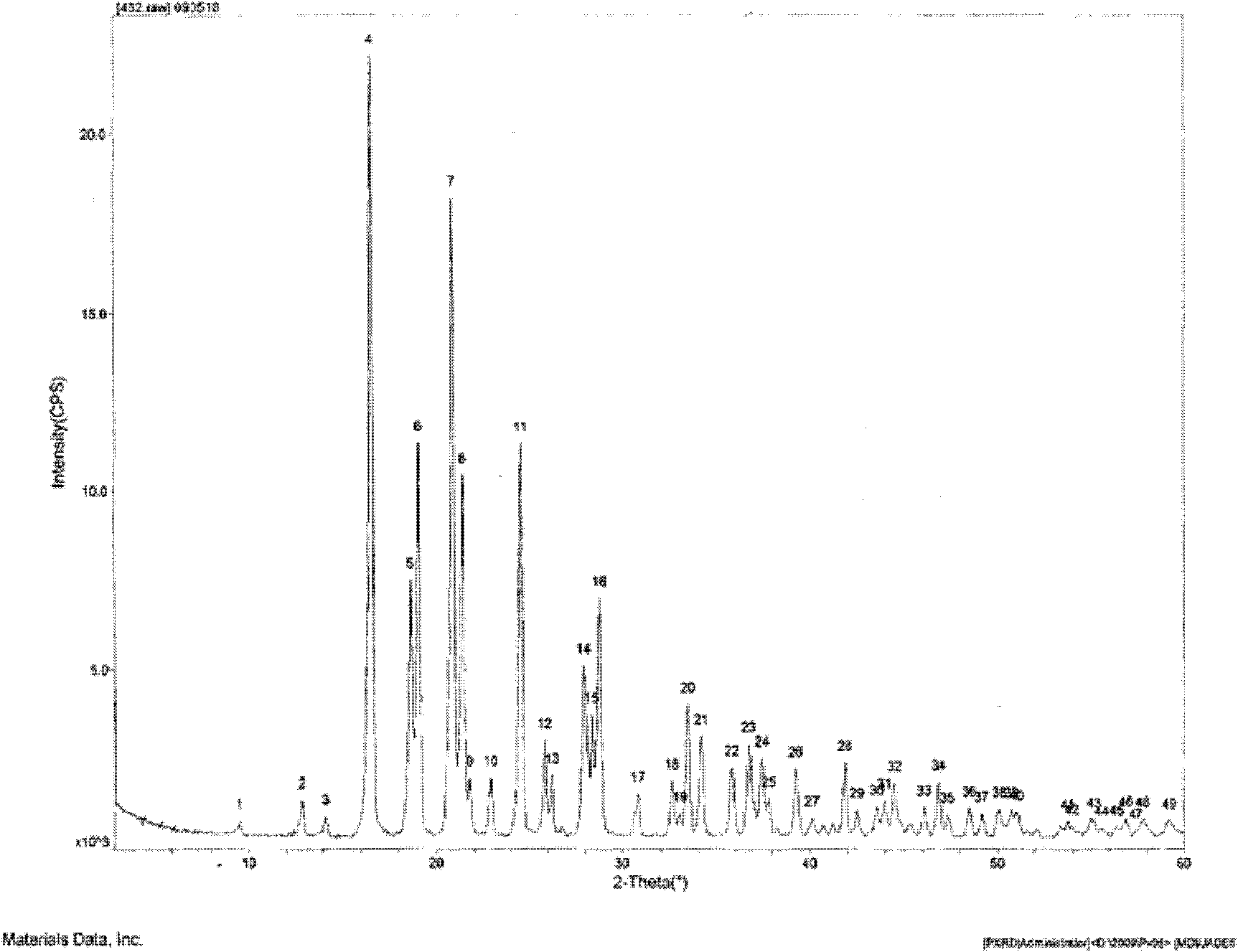
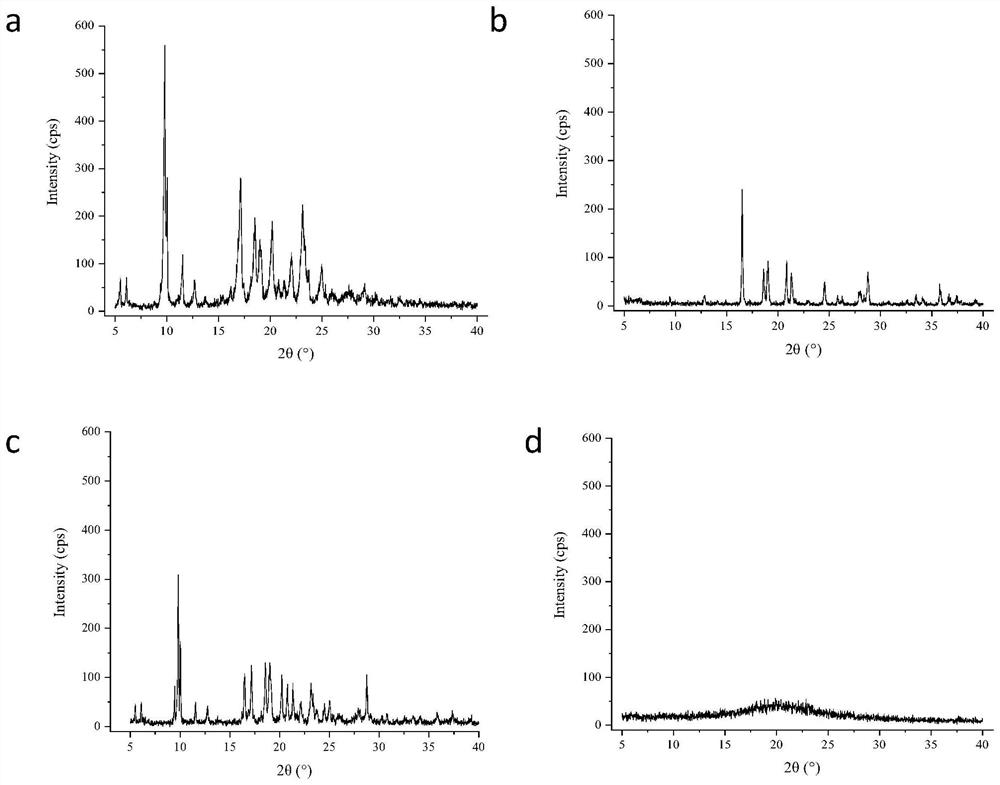
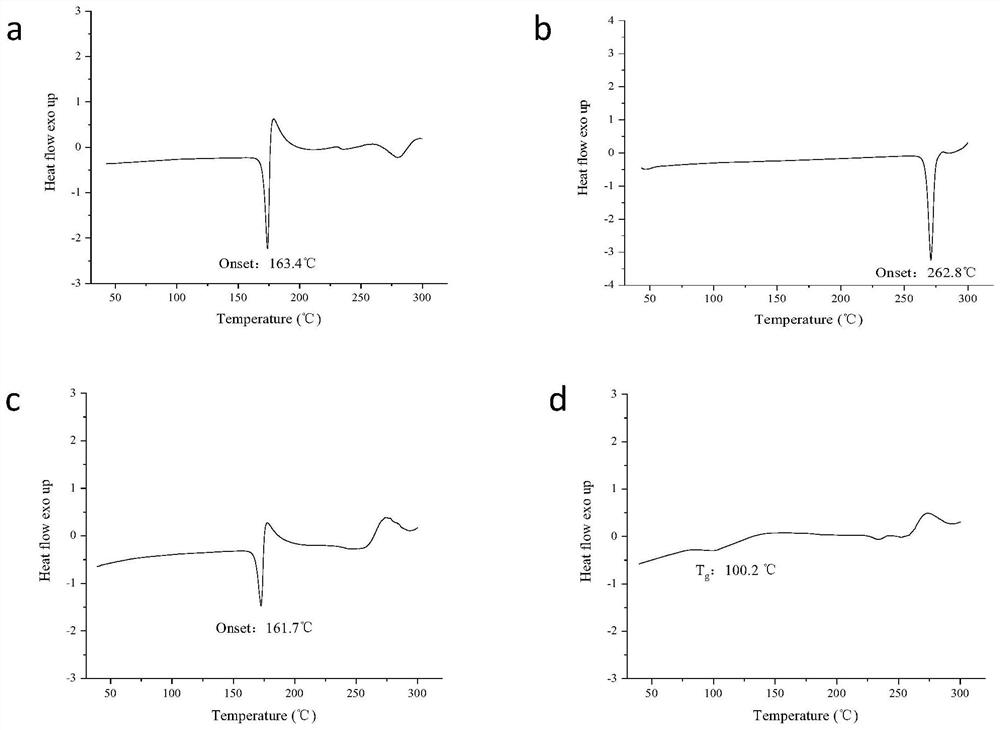
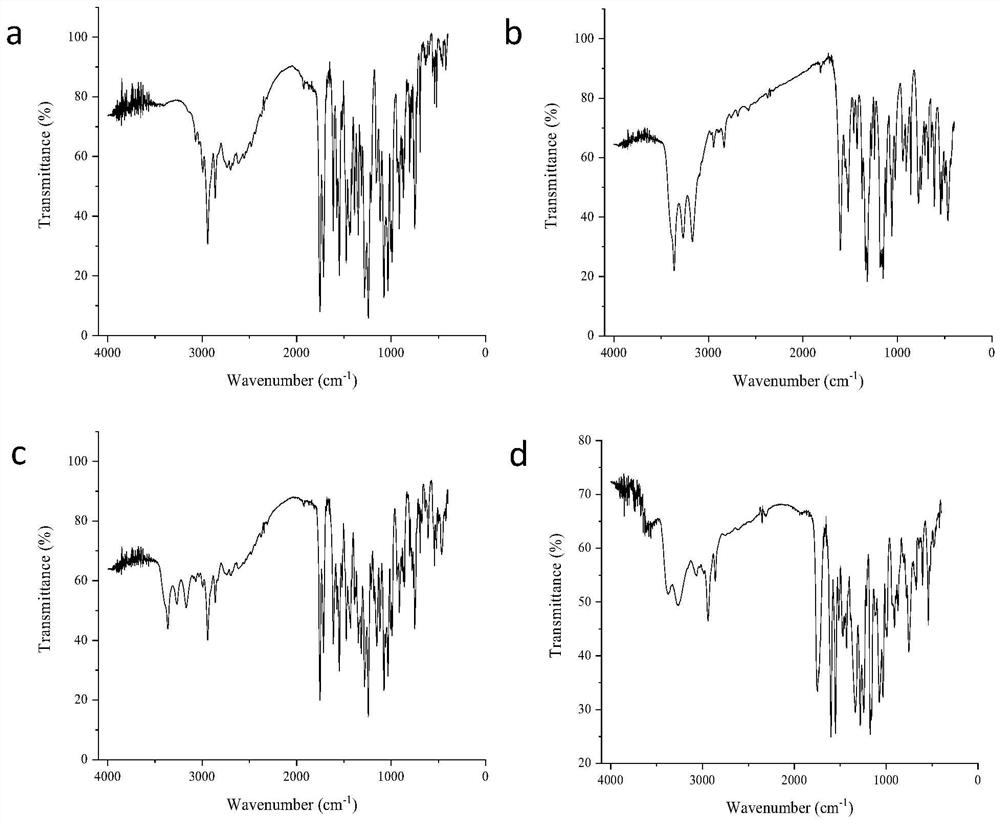
The invention discloses a candesartan cilexetil and hydrochlorothiazide co-amorphous substance formed by combining candesartan cilexetil and hydrochlorothiazide. According to a powder X-ray diffraction spectrum, a DSC spectrum and an infrared spectrum, the co-amorphous substance is a novel solid form completely different from each monomer and a physical mixture thereof. The candesartan cilexetil and the hydrochlorothiazide are used for preparing the co-amorphous substance, so that the solubility and the dissolution rate of the candesartan cilexetil can be significantly improved, and the co-amorphous substance is expected to become a new raw material solid form of a candesartan cilexetil / hydrochlorothiazide compound tablet and has good application development prospects.
Owner:CHINA PHARM UNIV
Drug capable of treating hypertension and preparation method thereof
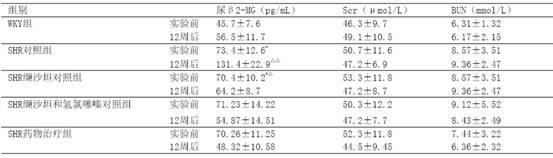
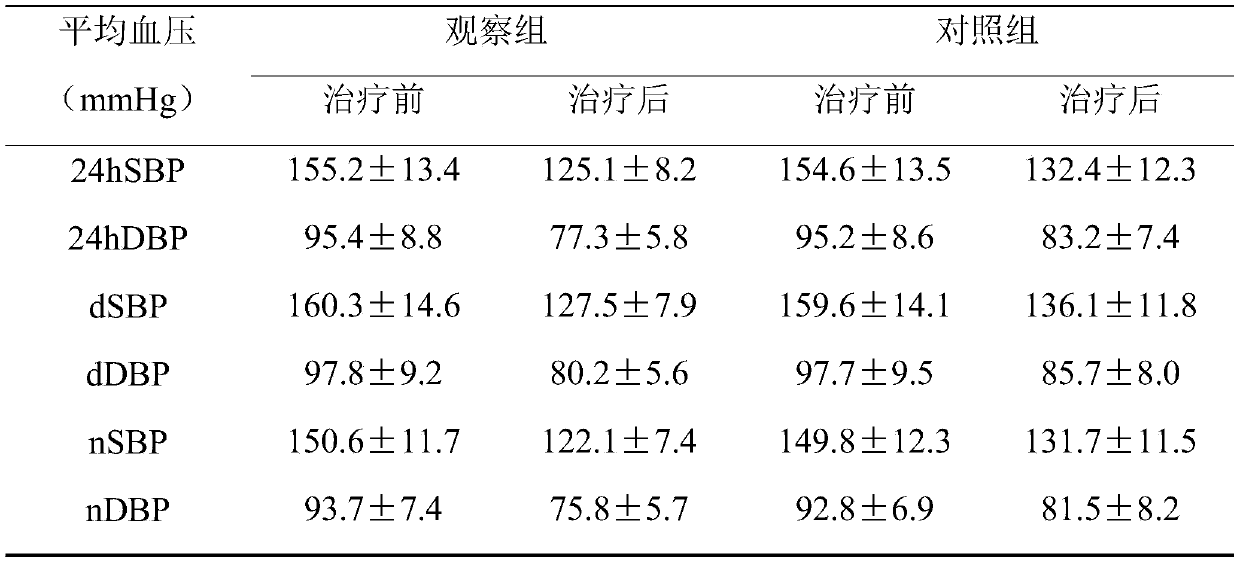
InactiveCN110755505AThe coefficient of variation of 24h blood pressure decreasedImproved blood pressure variabilityOrganic active ingredientsCapsule deliveryNifedipineHydrochlorothiazide
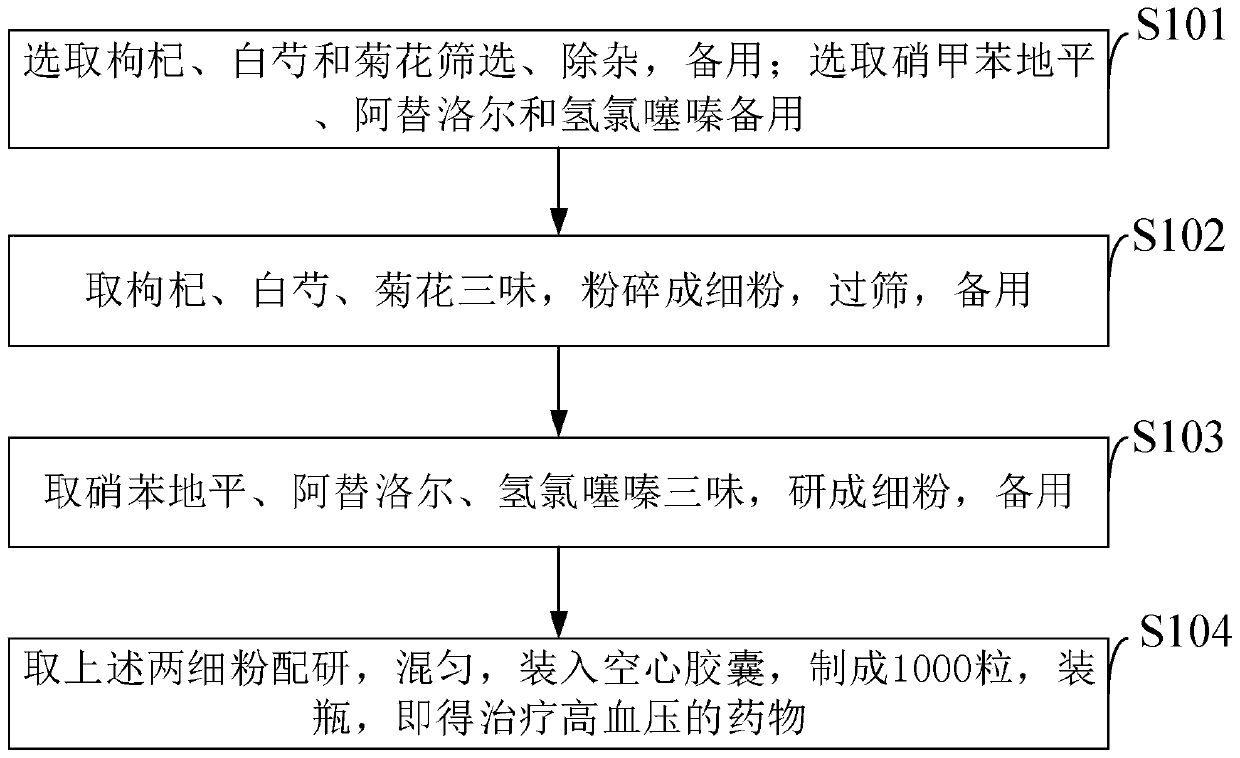
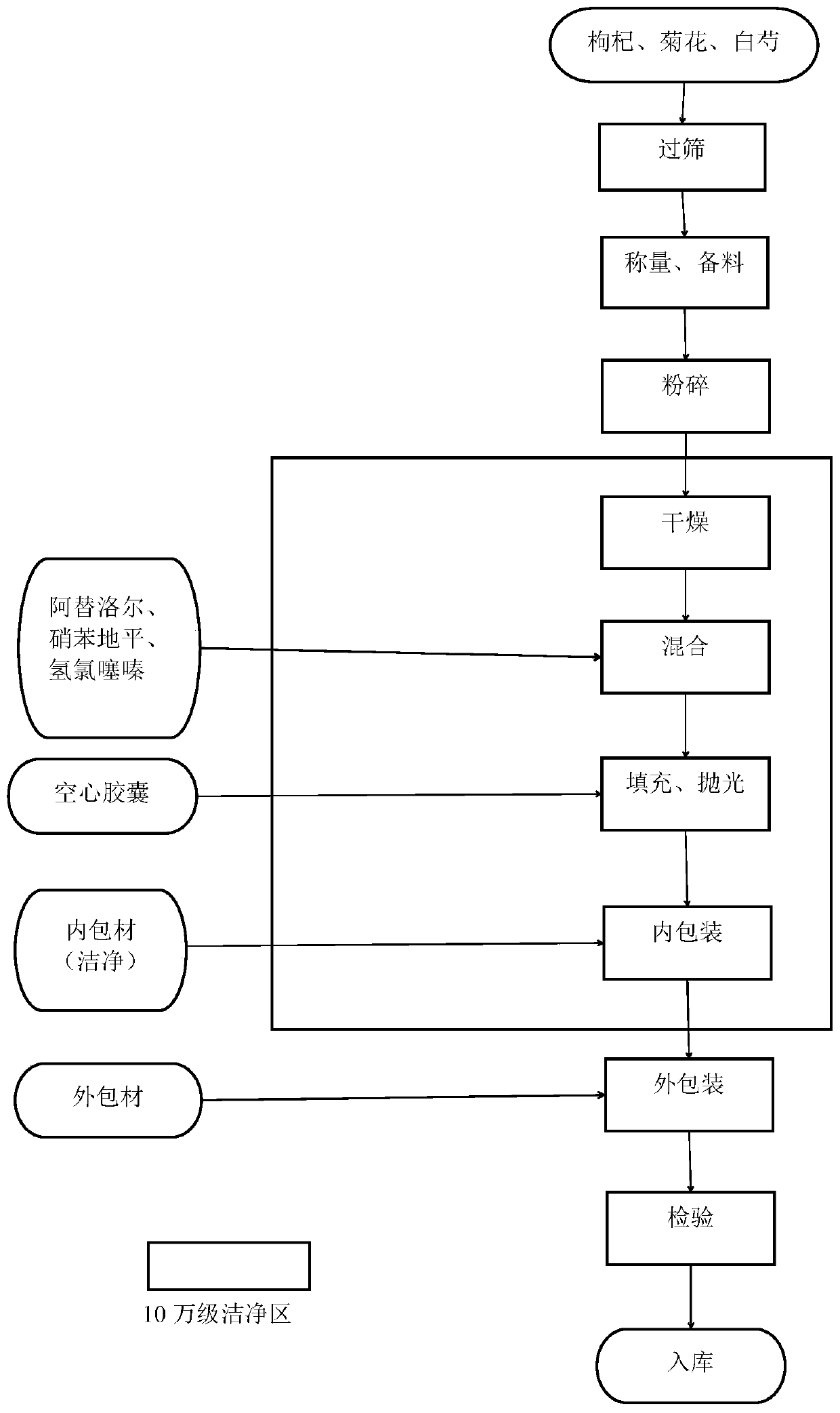
The invention belongs to the technical field of medicines, and discloses a drug capable of treating hypertension and a preparation method thereof. The drug capable of treating hypertension is composedof the following ingredients: 150 grams of Chinese wolfberry fruits, 250 grams of white peony, 100 grams of chrysanthemum flowers, 10.0 grams of Nifedipine, 12.5 grams of Atenolol, and 6.25 grams ofhydrochlorothiazide. The preparation method of the drug capable of treating hypertension disclosed by the invention comprises the following steps: screening the Chinese wolfberry fruits, the white peony and the chrysanthemum flowers, and removing impurities for standby application; selecting the Nifedipine, the Atenolol and the hydrochlorothiazide for standby application; drying the Chinese wolfberry fruits, the white peony and the chrysanthemum flowers, carrying out crushing so as to obtain fine powders, carrying out sieving, carrying out drying in a constant-temperature drying box at 95 DEGC for 15 hours, and carrying out sterilization for standby application; grinding the Nifedipine, the Atenolol and the hydrochlorothiazide into fine powders for standby application; blending the fine powders, carrying out grinding and sieving, carrying out uniform mixing in a mixing machine for 40 minutes so as to obtain a mixture, filling the mixture in hollow capsules so as to prepare 1000 filledcapsules, and carrying out bottling so as to obtain the drug capable of treating hypertension.
Owner:福建省南平市人民医院
Method for detecting ethanol and isopropanol in valsartan hydrochlorothiazide
PendingCN113945647AQuality assuranceEfficient detectionComponent separationPolyethylene glycolVapor phase chromatography
The invention belongs to the field of pharmaceutical analysis, and relates to a method for simultaneously detecting ethanol and isopropanol in a valsartan hydrochlorothiazide preparation, which adopts a gas chromatography method for detection, adopts a headspace sampling mode for sampling, takes nitrogen as carrier gas, adopts a chromatographic column with polyethylene glycol as a stationary phase, adopts a hydrogen flame ionization detector as a detector type, and adopts programmed heating. The detection method can effectively detect the content of ethanol and isopropanol in valsartan hydrochlorothiazide, has the advantages of high sensitivity, high degree of separation, good repeatability and durability, simple operation and stable and reliable result, can be used for controlling ethanol and isopropanol in valsartan hydrochlorothiazide, and provides an effective guarantee for the quality of a final finished product.
Owner:LUNAN PHARMA GROUP CORPORATION
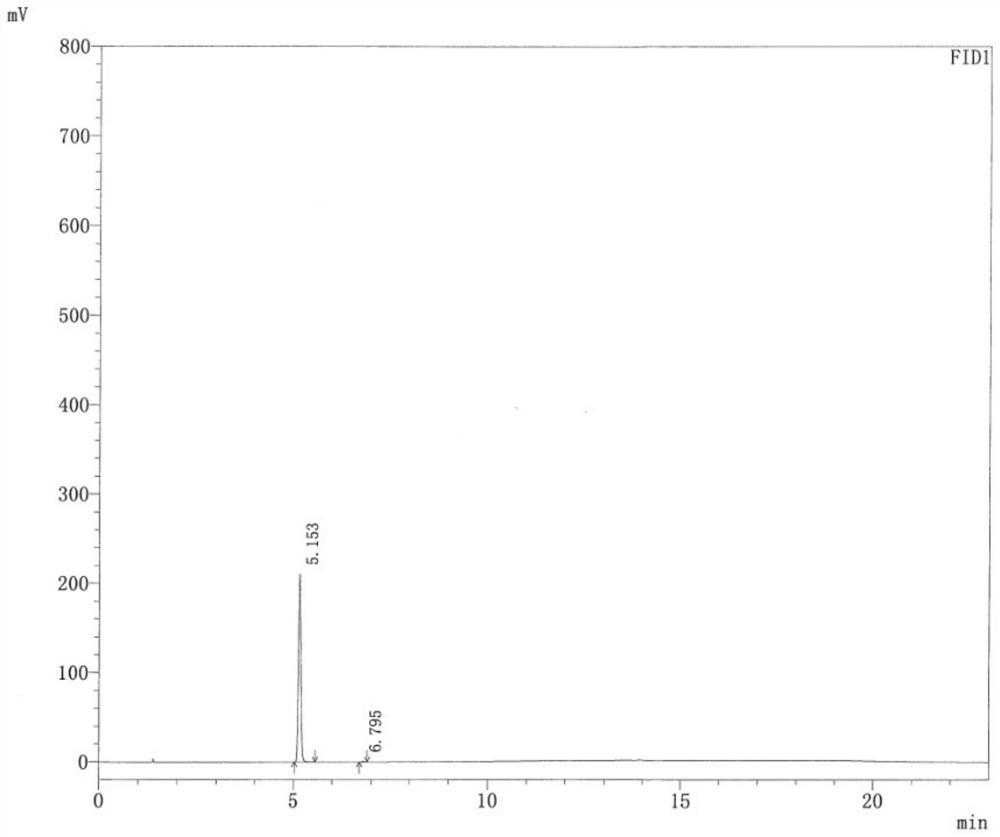
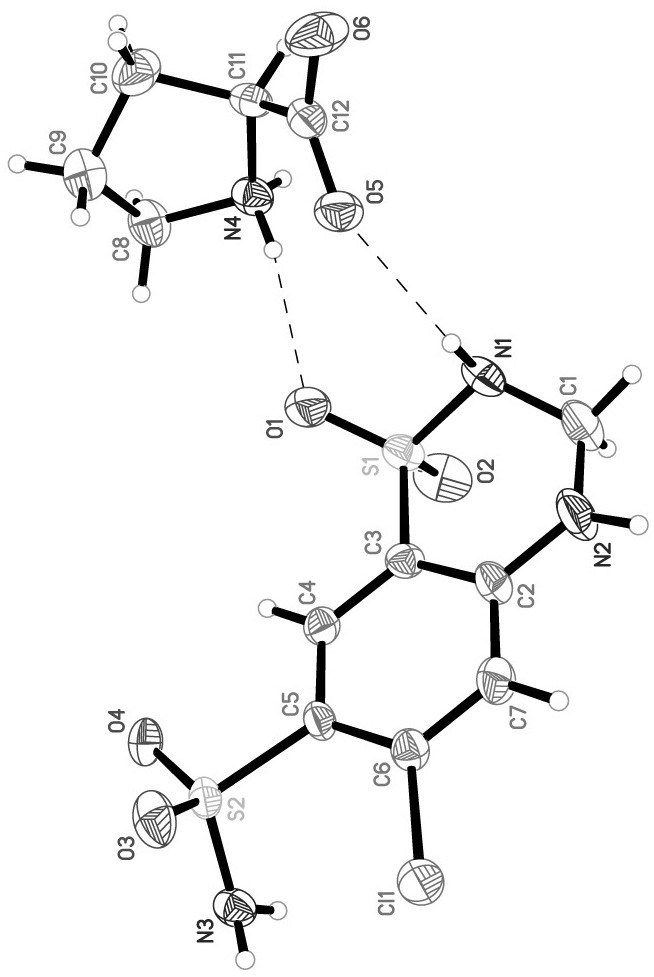
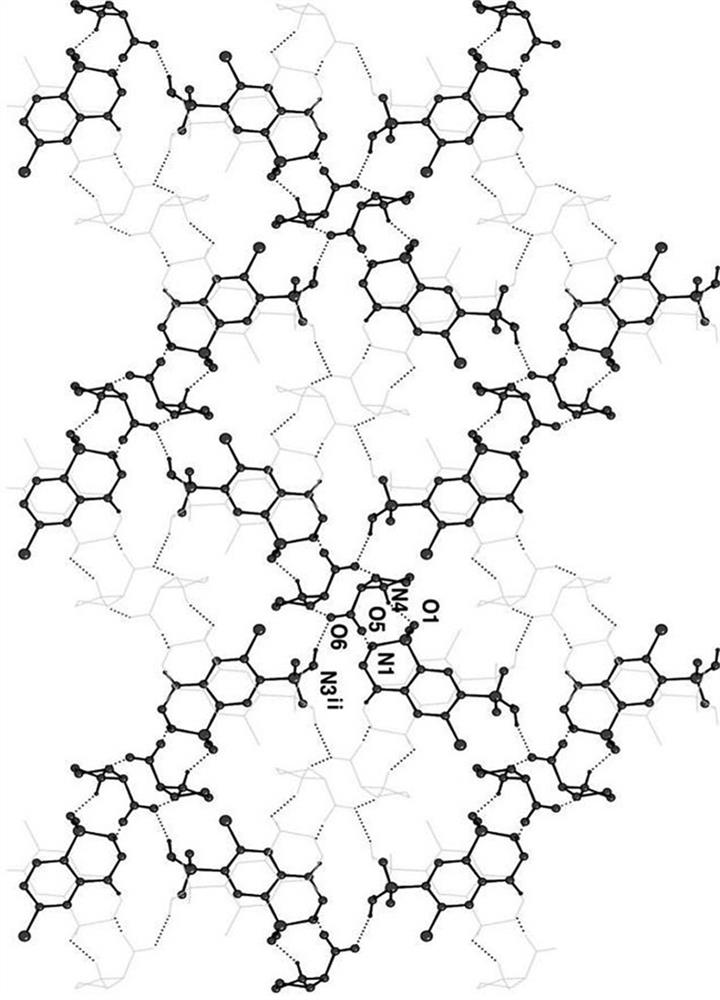
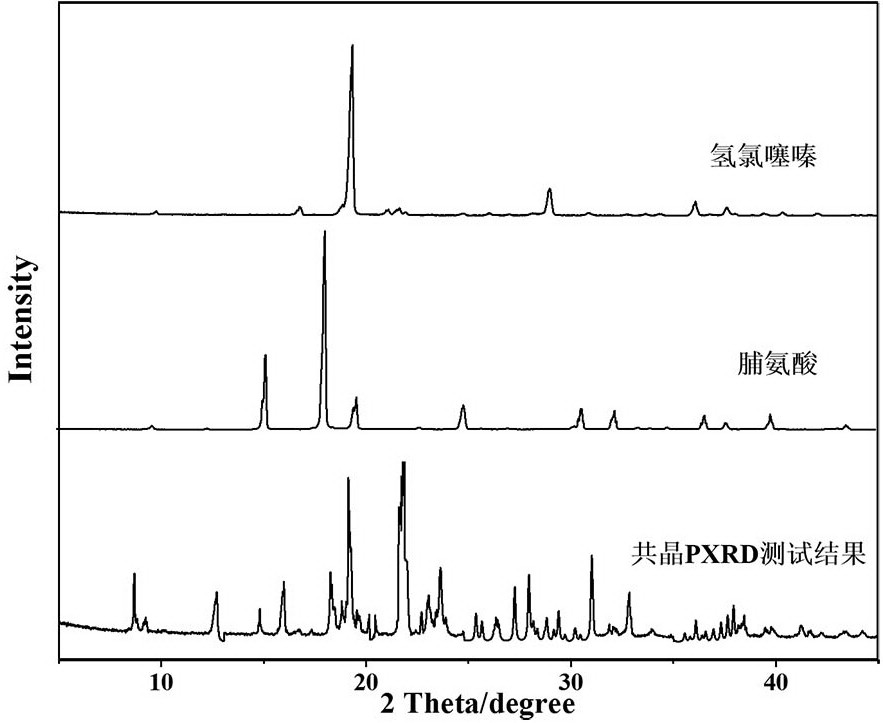
A kind of cocrystal of hydrochlorothiazide and proline and preparation method thereof
InactiveCN108440449BSimple manufacturing methodMild reaction conditionsOrganic chemistry methodsCrystal systemEfficacy
A drug co-crystal of hydrochlorothiazide drug and proline and a preparation method thereof, the invention relates to the field of drug co-crystal. The composition of this hydrochlorothiazide-proline cocrystal is [C 7 h 8 N 3 o 4 S 2 Cl·C 5 h 9 NO 2 ], the basic structural unit is constituted by a hydrochlorothiazide molecule and a proline molecule. The eutectic belongs to the orthorhombic crystal system, and the space group is P ca2 1 . Using hydrochlorothiazide and proline as raw materials, the drug eutectic is prepared by solvent evaporation and temperature reduction. The drug eutectic of the invention improves the solubility of hydrochlorothiazide, and lays a foundation for improving its bioavailability and drug efficacy. The preparation method of the drug co-crystal is simple and easy, has low cost and is convenient for large-scale production.
Owner:OCEAN UNIV OF CHINA
A kind of antihypertensive pharmaceutical composition
ActiveCN109528707BReduce releaseHydroxy compound active ingredientsPharmaceutical non-active ingredientsCholesterolDaidzein
The present invention discloses a hypotensive pharmaceutical composition. The composition is prepared by dissolving daidzein, honokiol, soybean phosphatidylcholine, cholesterol, DSPE-PEG in an organicsolvent, then preparing a liposome suspension by a membrane-sonic method, then adding hydrochlorothiazide, rhynchophylline and a diluent into the liposome suspension, performing freeze-dying, uniformly mixing the dried material with a disintegrant and a lubricant, and finally performing tabletting. The invention uses the daidzein and the honokiol to prepare the encapsulated liposome so as to delay the release rate of the drug, and the liposome can be released simultaneously with the hydrochlorothiazide and the rhynchophylline, thereby improving the coordination of the action of drug components in the body and increasing the curative effect.
Owner:HUBEI UNIV
Application of chlorothiazide in preparing medicament for inhibiting tumor cell metastasis and diffusion
InactiveCN104606200AAbility to inhibit metastasisReduce the number of deathsOrganic active ingredientsAntineoplastic agentsLymphatic SpreadNormal cell
Owner:南京格耀生物科技有限公司
Preparation method for compound losartan potassium-hydrochlorothiazide pharmaceutical composition
ActiveCN102475707BImprove stabilityAvoid contactOrganic active ingredientsPill deliveryCelluloseCross-link
The invention relates to a preparation method for a losartan potassium-hydrochlorothiazide tablet. The losartan potassium-hydrochlorothiazide tablet is characterized in that: after losartan potassium and starch are mixed, 10% of starch slurry is used to prepare particles with a suitable hardness; hydrochlorothiazide, cross linked sodium carboxymethyl cellulose and lactose are mixed, and particles with a proper hardness are prepared from an obtained mixture by using 5% of a polyvinylpyrrolidone K30 solution; the two kinds of particles and magnesium stearate are uniformly mixed and then are subjected to tabletting. According to the invention, a low dissolution rate caused by interaction among drugs in primary granulation is avoided, and an in vitro dissolution rate can be improved greatly, thereby enhancing bioavailability.
Owner:TIANJIN HANKANG PHARMA BIOTECH
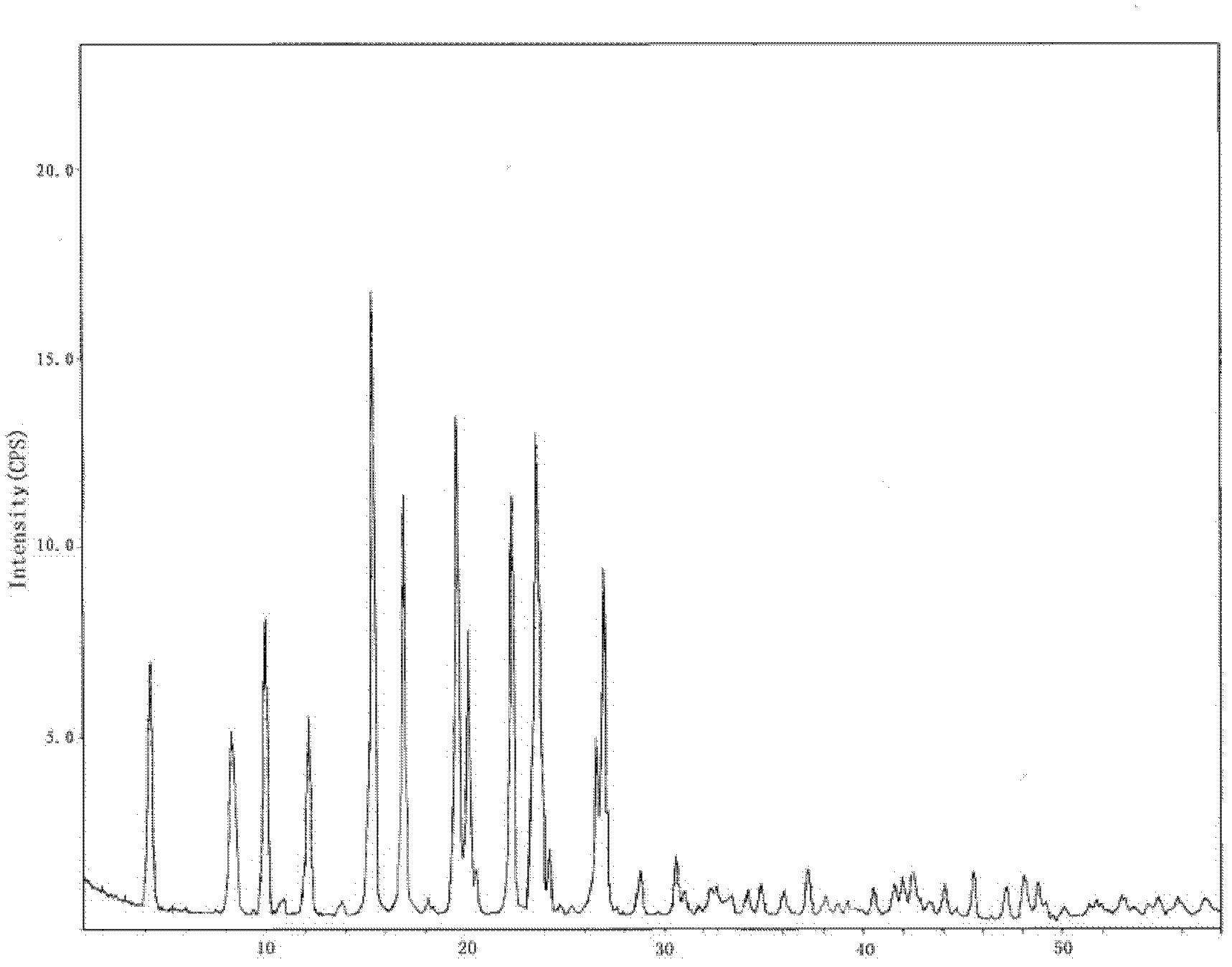
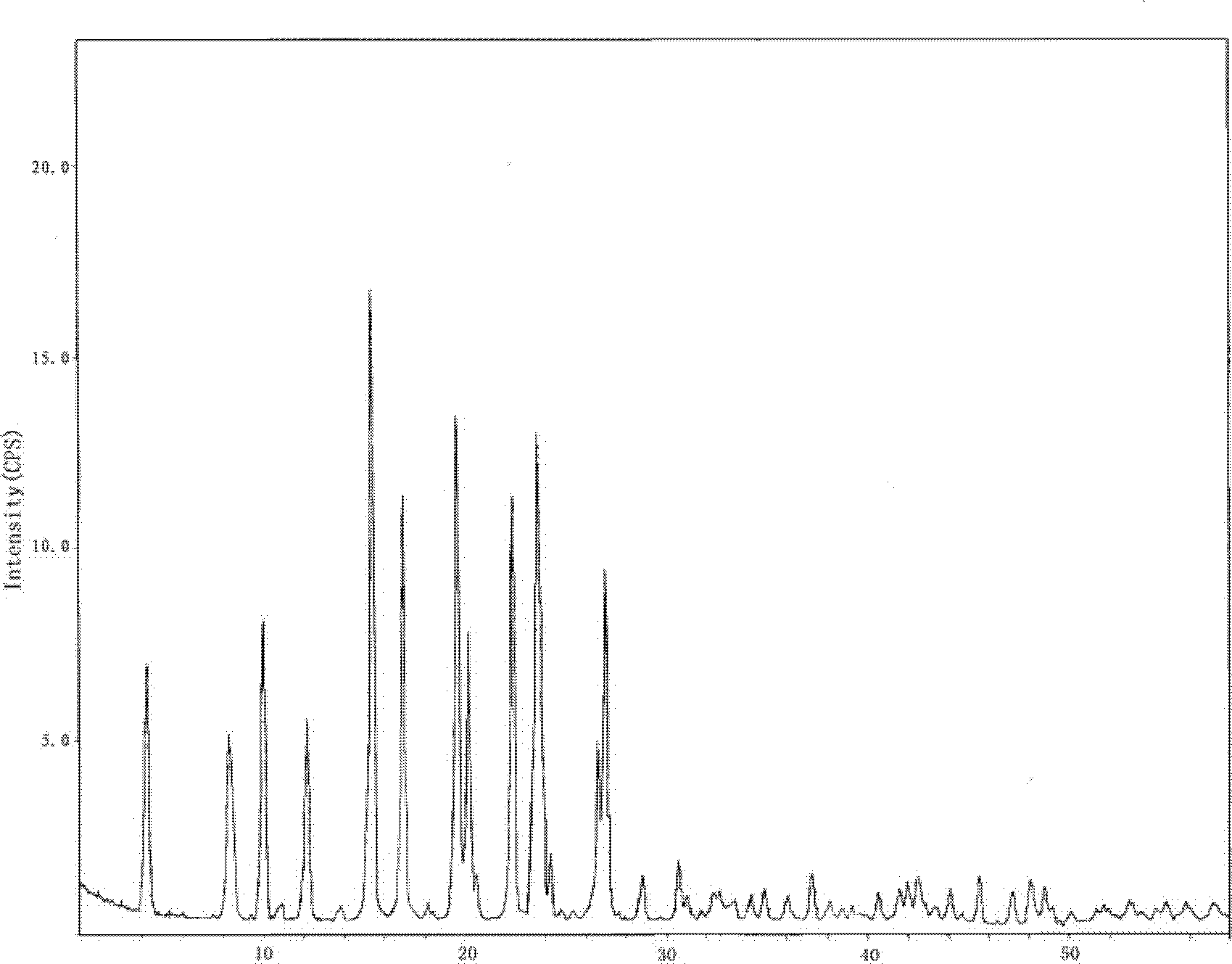
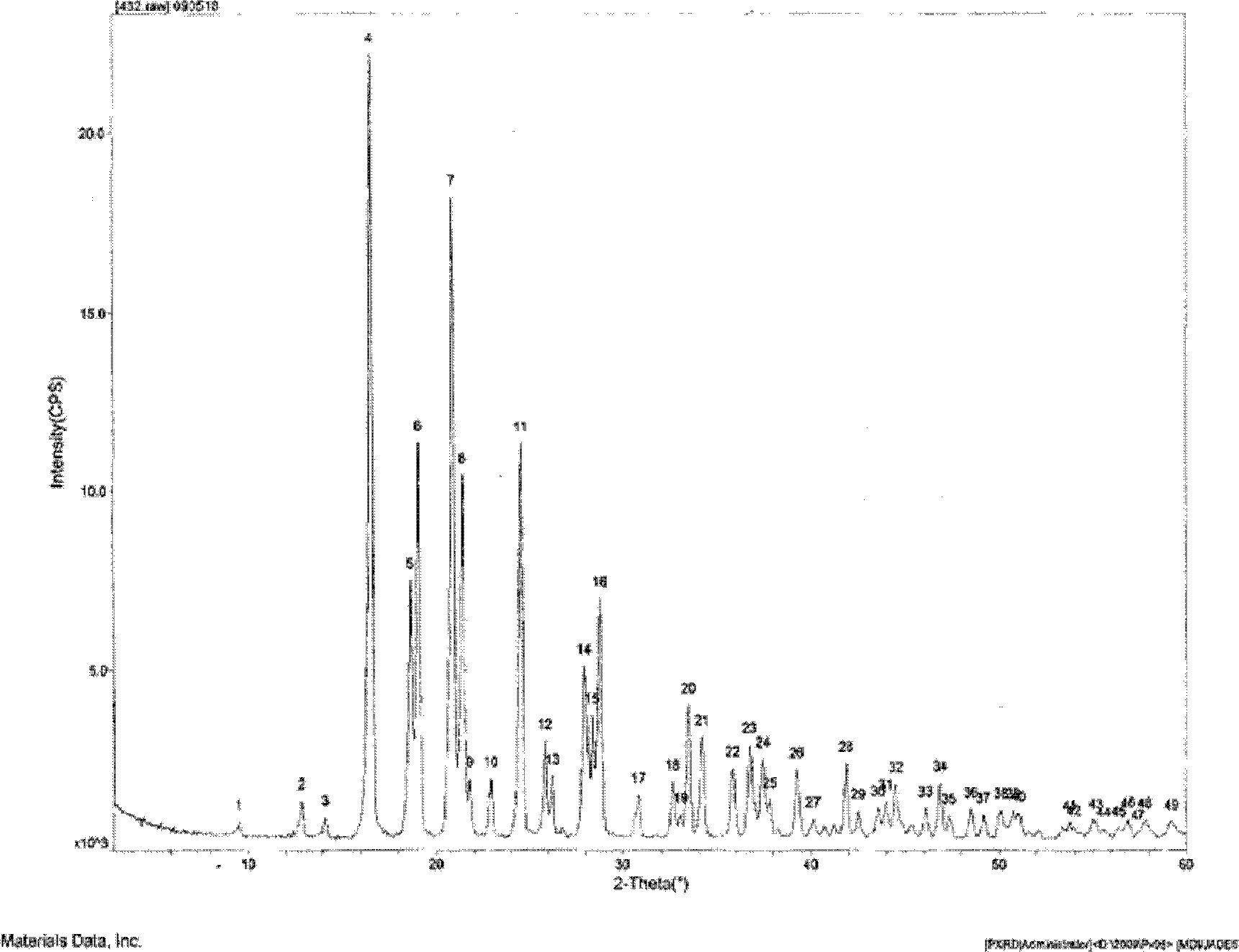
Hydrochlorothiazide crystal and candesartan cilexetil hydrochlorothiazide medicinal combination thereof
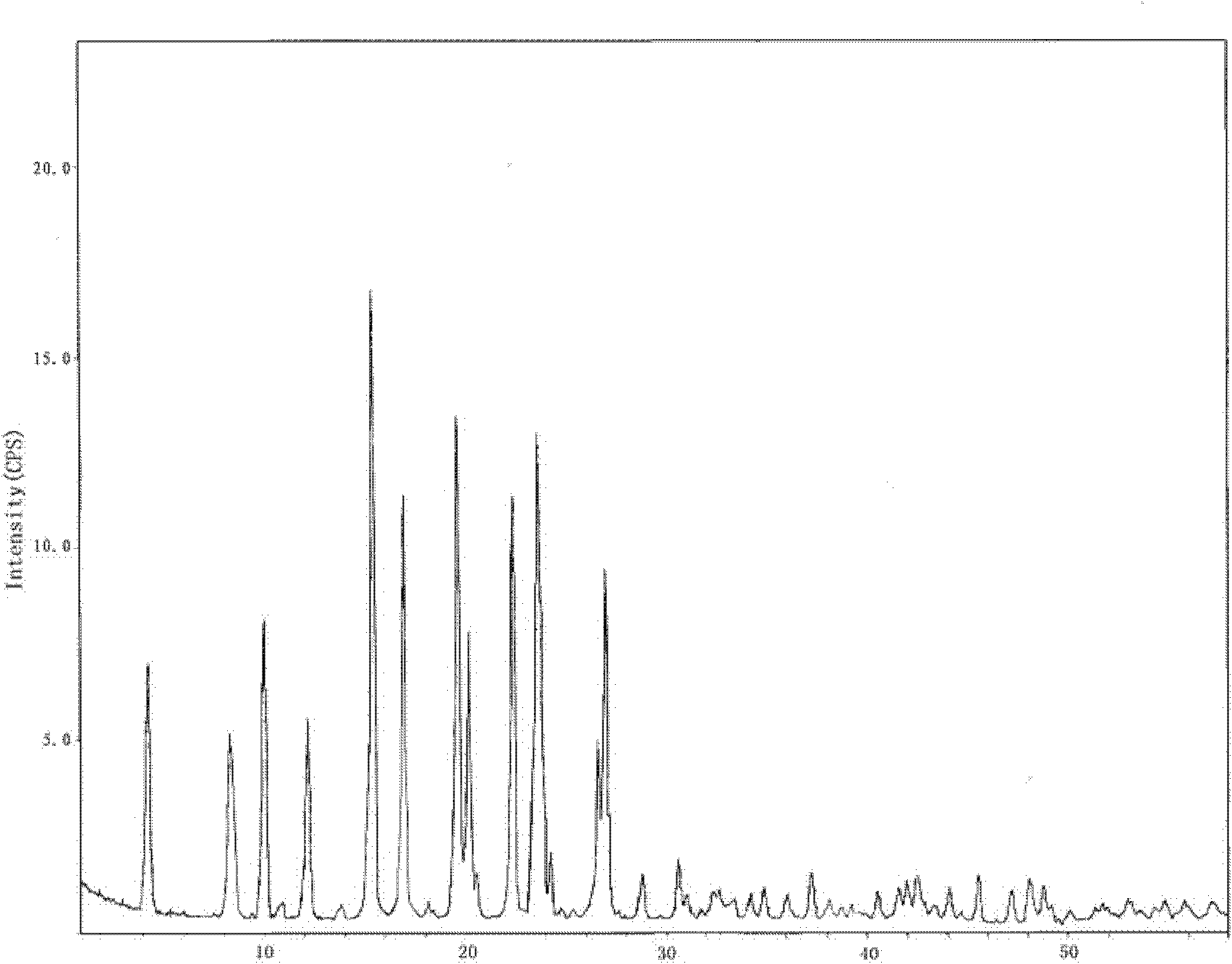
The invention discloses a hydrochlorothiazide crystal and a candesartan cilexetil hydrochlorothiazide medicinal combination thereof. The characteristic peaks of the hydrochlorothiazide crystal in an X-ray powder diffraction pattern, which are measured and obtained by using a Cu-K[alpha] ray, are displayed when 2[theta] is 4.1 degrees, 8.2 degrees, 9.8 degrees, 12.1 degrees, 15.1 degrees, 16.7 degrees, 19.3 degrees, 20.0 degrees, 22.1 degrees, 23.3 degrees and 26.8 degrees. The combination comprises 4-20 parts of candesartan cilexetil, 10-15 parts of the hydrochlorothiazide crystal, 10-50 parts of pregelatinized starch, 15-35 parts of microcrystalline cellulose PH102, 10-45 parts of crosslinked polyvinylpyrrolidone and 0.5-1 part of magnesium stearate. The medicinal combination has a reasonable prescription, stable and reliable quality, and better disintegration time limit and dissolution rate; a direct powder tabletting process is adopted; the process is simple; the production period is short; the production cost is low; and the industrialized production is facilitated.
Owner:HAINAN JINRUI PHARMA CO LTD
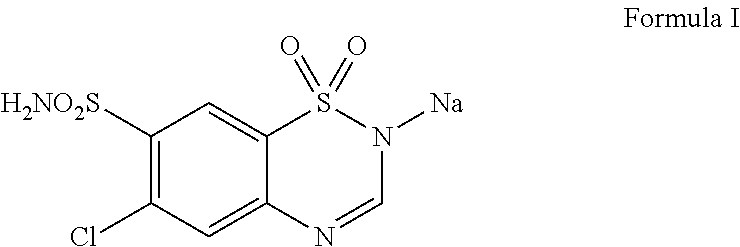
Injectable composition containing chlorothiazide
InactiveUS20150320680A1Pharmaceutical delivery mechanismPharmaceutical non-active ingredientsReady to usePharmacology
The present invention relates to stable ready to use injectable liquid composition of chlorothiazide or its pharmaceutically acceptable salts.
Owner:GETZ PHARMA RES PVT
A kind of synthetic method of hydrochlorothiazide
ActiveCN108658896BReduce the difficulty of refiningHigh purityOrganic chemistryOrganic acidHydrochlorothiazide
The invention relates to a method for synthesizing hydrochlorothiazide. A method for synthesizing hydrochlorothiazide comprises the following steps: using chlorothiazide as a reactant, under the action of an organic acid and a hydroboration reagent, a reduction reaction occurs to generate crude hydrochlorothiazide. Using borohydride as a reducing agent, the carbon-nitrogen double bond in chlorothiazide is reduced to generate hydrochlorothiazide, without the participation of formaldehyde or similar substances in the whole process, so it is safer and more environmentally friendly than the existing synthetic methods, and the present invention has advantages in yield and Purity has been improved.
Owner:CHANGZHOU PHARMA FACTORY
Hydrochlorothiazidum chewable tablets for dog or cat
InactiveCN101190202ABreak through the shortcomings of poor palatabilityHigh cure rateOrganic active ingredientsPill deliveryDiseaseOral glucose
The invention discloses a hydrochlorothiazide chewable tablet used for dogs and cats. The invention can overcome the disadvantage of the poor palatability of the existing tablets, promote dogs and cats to chew and adsorb fully, effectively ensure the dosage of administration, enhance the cure rate of feline or canine diseases and reduce the waste of medicine. The tablet of the invention includes the following components represented by weight-percentage respectively: 1-5 percent of aspartame, 20-30 percent of the mixture of oral glucose and skim milk powder, in which the ratio of oral glucose and skim milk powder is 1:1-1:4, 40-60 percent of excipient, 0.5-1 percent of glidant and 30-50 percent of hydrochlorothiazide.
Owner:TIANJIN RINGPU BIO TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com