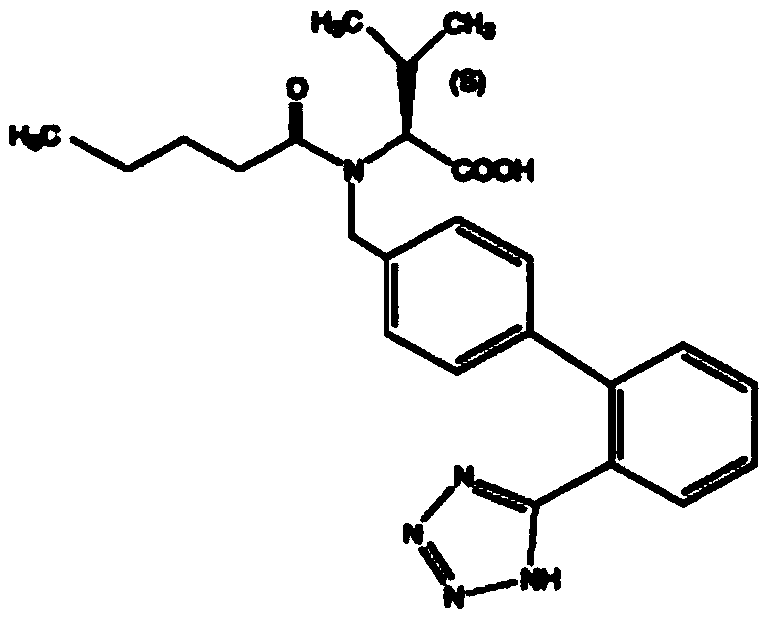
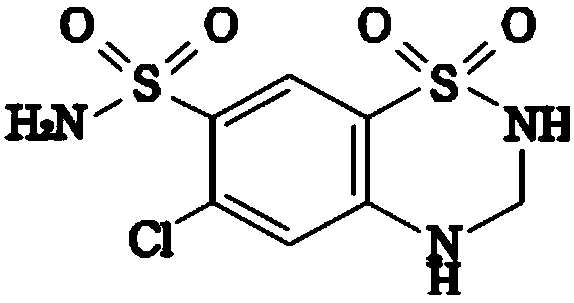
A kind of valsartan hydrochlorothiazide tablet and preparation method thereof
A technology for chlorothiazide tablets and hydrochlorothiazide, applied in the field of medicine, can solve the problems of low in vitro dissolution rate, low bioavailability, increased degradation products and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Embodiment 1, preparation valsartan / hydrochlorothiazide sheet
[0047] The present embodiment provides the prescription and preparation method for preparing 1000 valsartan / hydrochlorothiazide tablets, as follows:
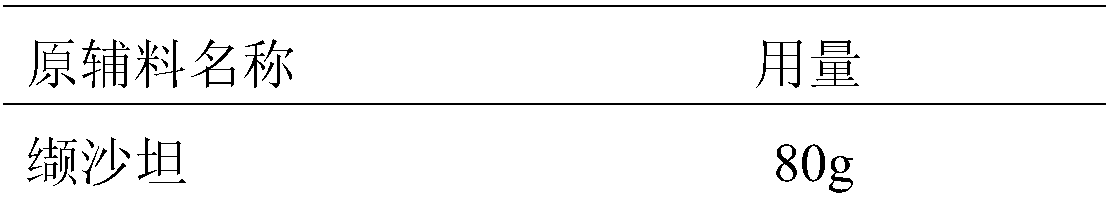
[0048] Prescription composition:
[0049]
[0050]
[0051] Control the ambient humidity RH≤40%.
[0052] The preparation method comprises the following steps:
[0053] 1) Grinding hydrochlorothiazide to obtain hydrochlorothiazide powder (particle size range D90≤130 μm), passing crospovidone XL through a 40-mesh sieve, and magnesium stearate (additional) passing through a 80-mesh sieve;
[0054] 2) Weigh valsartan, crospovidone XL, copovidone S630 (internal addition), magnesium stearate (internal addition), hydrochlorothiazide, colloidal silicon dioxide, microcrystalline cellulose PH102 and mix in wet method Mix in the granulator for 2 minutes (stirring 3r / s, shearing 3r / s) and pass through a 40-mesh sieve to disperse;
[0055] 3) Pre-mixing: put the ...
Embodiment 2
[0062] Embodiment 2, preparation valsartan / hydrochlorothiazide sheet
[0063] The present embodiment provides the prescription and preparation method for preparing 1000 valsartan / hydrochlorothiazide tablets, as follows:
[0064] Prescription composition:
[0065]
[0066] The preparation method is basically the same as in Example 1.
Embodiment 3
[0067] Embodiment 3, preparation valsartan / hydrochlorothiazide sheet
[0068] The present embodiment provides the prescription and preparation method for preparing 1000 valsartan / hydrochlorothiazide tablets, as follows:
[0069] Prescription composition:
[0070]
[0071]
[0072] The preparation method is basically the same as in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com