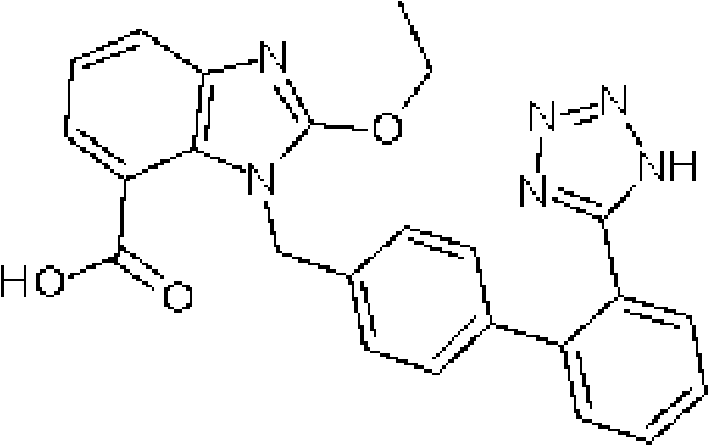
Hydrochlorothiazide crystal and candesartan cilexetil hydrochlorothiazide medicinal combination thereof
A technology of candesartan cilexetil and hydrochlorothiazide, which is applied in the field of medicine and can solve problems such as poor bioavailability, slow dissolution of hydrochlorothiazide, and unsatisfactory curative effect of pharmaceutical preparations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0084] The preparation of embodiment 1 hydrochlorothiazide crystal
[0085] (1) 1kg hydrochlorothiazide is dissolved in acetone to obtain acetone solution whose concentration is 0.1g / ml hydrochlorothiazide;
[0086] (2) Add distilled water dropwise to the acetone solution under stirring at 160r / min until the solution becomes turbid;
[0087] (3) under the ultrasonic field that power is 0.5KW, flow the organic mixed solution of ethanol and ether in the solution gained in step 2, continue the stirring of 25r / min; Wherein the volume ratio of ethanol and ether in the organic mixed solution is 5: 6, The volume ratio of described mixed solution and acetone is 1: 1;
[0088] (4) Continue ultrasonication for 2 minutes, let stand, grow crystals at 16° C. for 2 hours, filter, wash the filter cake with ether, and vacuum-dry to obtain hydrochlorothiazide crystals.
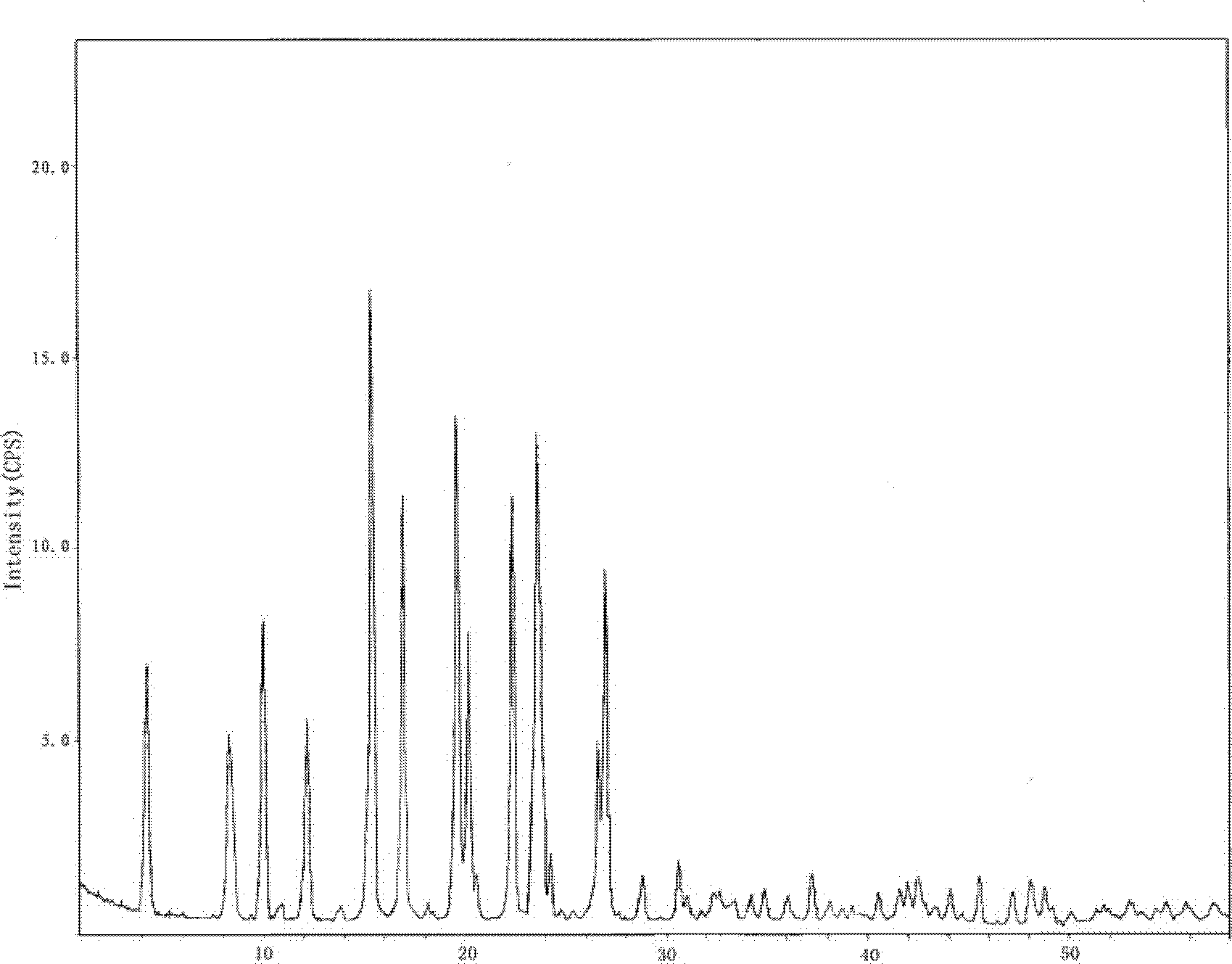
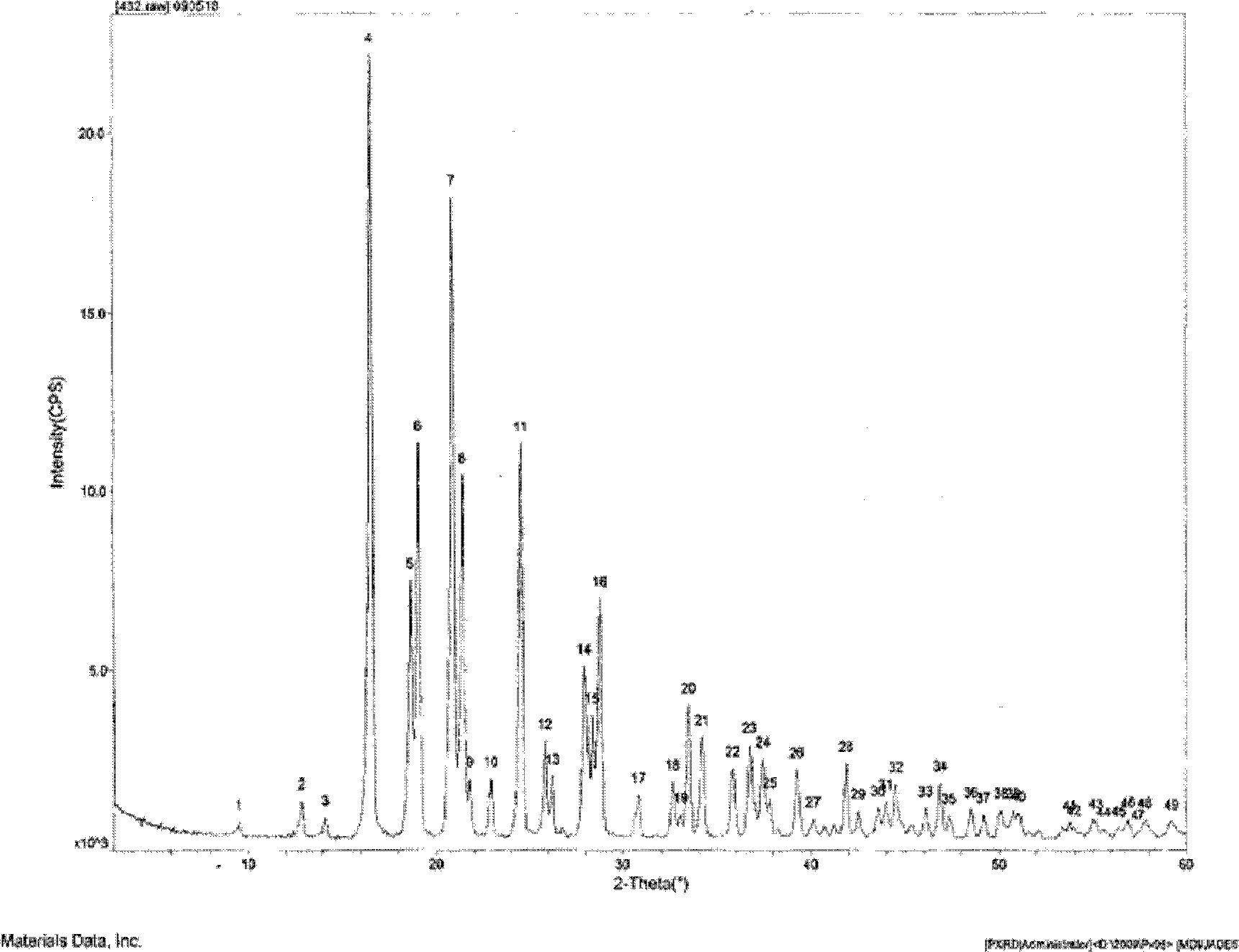
[0089] Such as figure 1 As shown, the characteristic peaks in the X-ray powder diffraction pattern obtained by measuring...
Embodiment 2
[0090] The preparation of embodiment 2 hydrochlorothiazide crystals
[0091] (1) 1kg hydrochlorothiazide is dissolved in acetone to obtain acetone solution whose concentration is 0.2g / ml hydrochlorothiazide;
[0092] (2) Add distilled water dropwise to the acetone solution under stirring at 120r / min until the solution becomes turbid;
[0093] (3) under the ultrasonic field that power is 0.4KW, flow the organic mixed solution of ethanol and ether in the solution gained in step 2, continue the stirring of 20r / min; Wherein the volume ratio of ethanol and ether in the organic mixed solution is 2: 3, The volume ratio of described mixed solution and acetone is 4: 5;
[0094] (4) Continue ultrasonication for 2 minutes, let stand, grow crystals at 12° C. for 1.5 hours, filter, wash the filter cake with ether, and vacuum-dry to obtain hydrochlorothiazide crystals.
[0095] Such as figure 1 As shown, the characteristic peaks in the X-ray powder diffraction pattern obtained by measur...
Embodiment 3
[0096] The preparation of embodiment 3 hydrochlorothiazide crystals
[0097] (1) 1kg hydrochlorothiazide is dissolved in acetone to obtain a solution of acetone with a concentration of 0.08g / ml hydrochlorothiazide;
[0098] (2) Add distilled water dropwise to the acetone solution under stirring at 180r / min until the solution becomes turbid;
[0099] (3) under the ultrasonic field that power is 0.6KW, flow the organic mixed solution of ethanol and ether in the solution gained in step 2, continue the stirring of 30r / min; Wherein the volume ratio of ethanol and ether in the organic mixed solution is 7: 6, The volume ratio of described mixed solution and acetone is 8:5;
[0100] (4) Continue ultrasonication for 3 minutes, let stand, grow crystals at 18° C. for 2.5 hours, filter, wash the filter cake with ether, and vacuum-dry to obtain hydrochlorothiazide crystals.
[0101] Such as figure 1 As shown, the characteristic peaks in the X-ray powder diffraction pattern obtained by ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| angle of repose | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com