Compound composition for treatment of high blood pressure and preparation method thereof
A composition and high blood pressure technology, applied in the field of medicine, can solve problems such as complicated process and unsuitable for large-scale industrial production, and achieve the effects of simplifying the preparation process, stabilizing the quality of preparations, and reducing manufacturing costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach 1
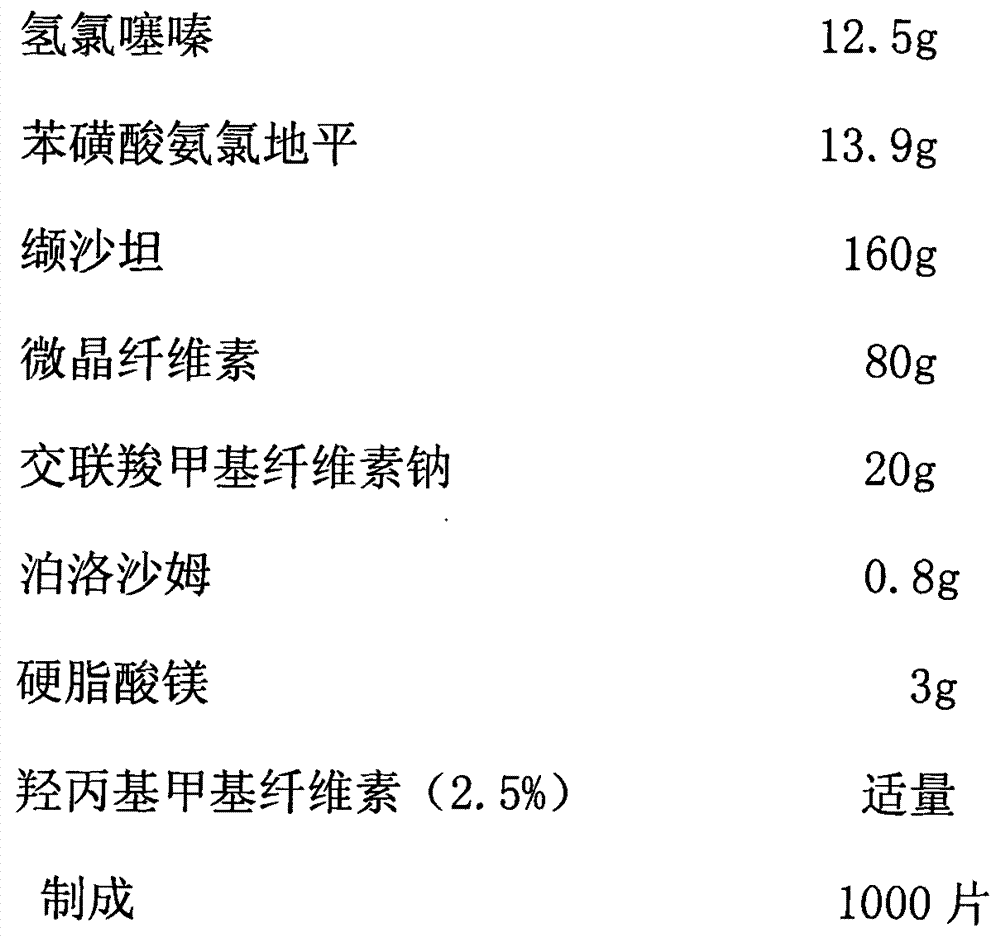
[0031]
[0032] The preparation method adopts wet granulation, and the process includes:
[0033] Step 1: The main drug fine powder and auxiliary material fine powder according to the prescription amount, the main drug hydrochlorothiazide and amlodipine besylate are mixed with croscarmellose sodium (3 / 4 of the prescription amount) and passed through a 40-mesh sieve , then add microcrystalline cellulose equivalent to the weight of the above-mentioned mixed raw and auxiliary materials, pass through a 40-mesh sieve after mixing, then add the main drug valsartan equivalent to its weight, pass through a 40-mesh sieve after mixing, and then add the remaining microcrystalline After the cellulose and valsartan are mixed, pass through a 40-mesh sieve twice.
[0034] Step 2: Add an appropriate amount of 1.5% hydroxypropyl methylcellulose solution to the mixed fine powder obtained in step 1, prepare wet granules by passing through a 20-mesh sieve, and dry them by blasting at 45 ° C. T...
Embodiment approach 2
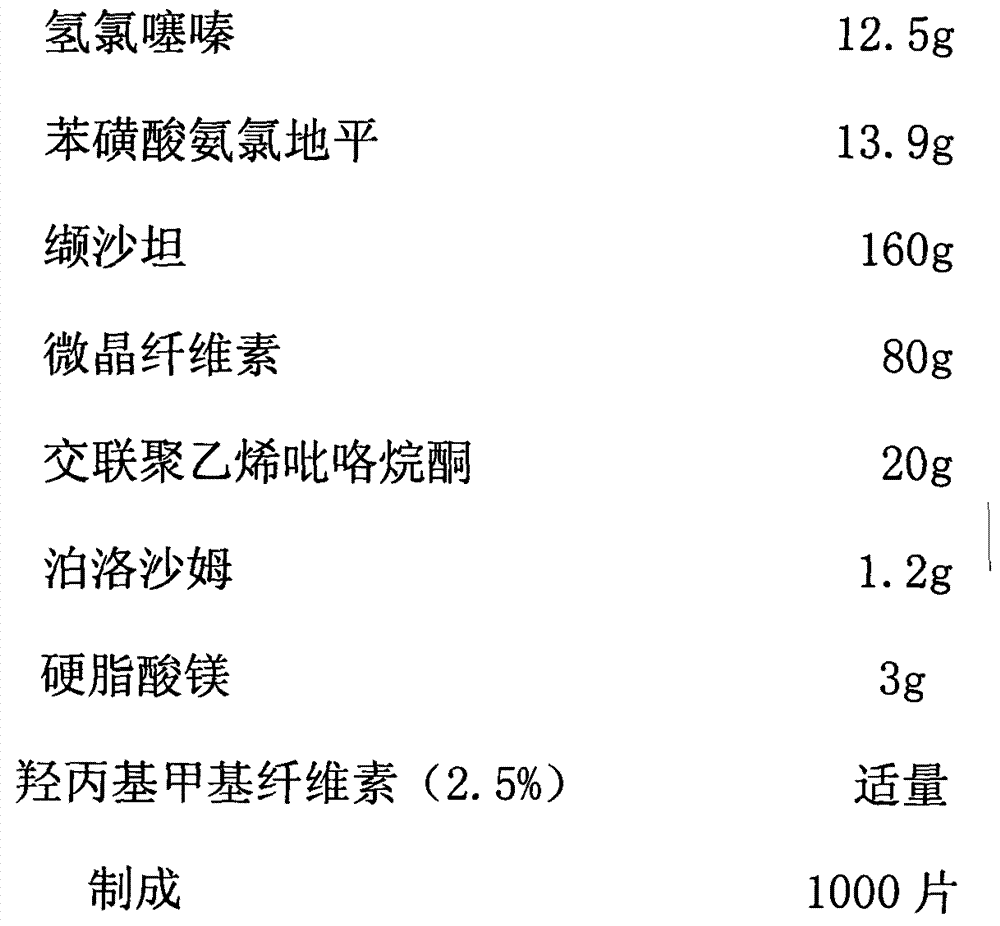
[0038]
[0039] The preparation method adopts wet granulation, and the process includes:
[0040] Step 1: The main drug fine powder and auxiliary material fine powder according to the prescription amount, the main drug hydrochlorothiazide and amlodipine besylate and cross-linked polyvinylpyrrolidone (3 / 4 of the prescription amount) are mixed and passed through a 40-mesh sieve, and then added Microcrystalline cellulose equivalent to the weight of the above mixed raw and auxiliary materials, after mixing, pass through a 40-mesh sieve, then add the main drug valsartan, which is equivalent to its weight, pass through a 40-mesh sieve after mixing, then add the remaining microcrystalline cellulose and After valsartan is mixed, pass through a 40-mesh sieve twice.
[0041] Step 2: Add an appropriate amount of 1.5% hydroxypropyl methylcellulose solution to the mixed fine powder obtained in step 1, prepare wet granules by passing through a 20-mesh sieve, and dry them by blasting at 4...
Embodiment approach 3
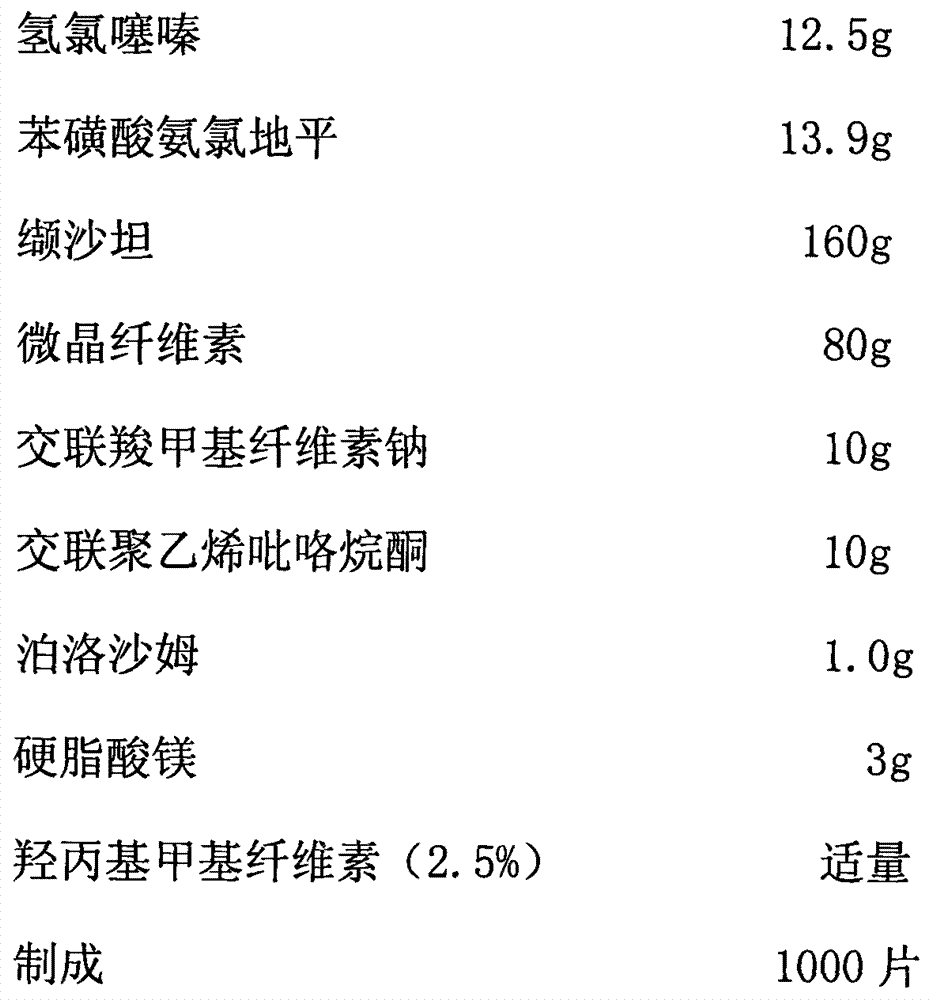
[0045]
[0046] The preparation method adopts wet granulation, and the process includes:
[0047] Step 1: the main drug fine powder and auxiliary material fine powder according to the prescription amount, the main drug hydrochlorothiazide and amlodipine besylate are mixed with cross-linked polyvinylpyrrolidone and passed through a 40-mesh sieve, and then add the equivalent weight of the above mixed raw materials Microcrystalline cellulose, after mixing, pass through a 40-mesh sieve, then add the main drug valsartan equivalent to its weight, pass through a 40-mesh sieve after mixing, then add the remaining microcrystalline cellulose and valsartan, mix and pass through twice 40 mesh screen.
[0048] Step 2: Add an appropriate amount of 1.5% hydroxypropyl methylcellulose solution to the mixed fine powder obtained in step 1, prepare wet granules by passing through a 20-mesh sieve, and dry them by blasting at 45 ° C. The moisture content of the granules is controlled at 2%-3%. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com