Compound telmisartan hydrochlorothiazide pharmaceutical composition and preparation method thereof
A technology of telmisartan and hydrochlorothiazide, applied in the field of telmisartan hydrochlorothiazide pharmaceutical composition and its new preparation, can solve the problems of low dissolution rate of hydrochlorothiazide
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
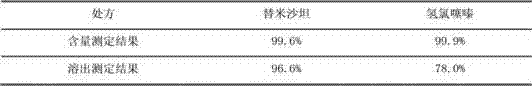
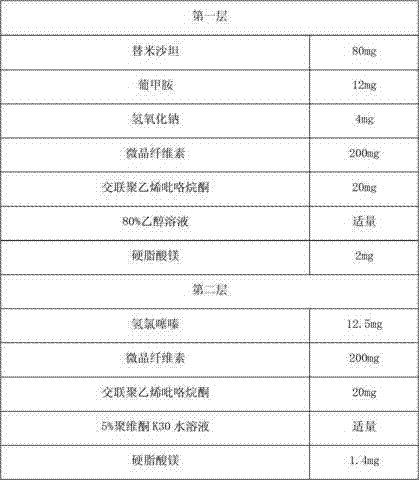
[0078] A compound telmisartan hydrochlorothiazide pharmaceutical composition, the described compound telmisartan hydrochlorothiazide pharmaceutical composition of every 1000, its formula consists of:
[0079] Inner Tablet Prescription
[0080]
[0081]
[0082] Outer Tablet Prescription
[0083]
[0084] Inner Tablet Coating Solution Prescription
[0085]
[0086] Preparation Process:
[0087] 1) Preparation of the inner layer tablet: After crushing hydrochlorothiazide through a 100-mesh sieve, place it in a rapid mixer with microcrystalline cellulose, crospovidone, and pregelatinized starch, and mix rapidly for 3-5 minutes, then add appropriate amount of water, Make soft material, pass through 20-mesh sieve, dry at 50±5℃, granulate with 20-mesh sieve, add sucrose fatty acid ester, and press into tablets.
[0088] 2) Inner layer tablet coating: Weigh the Eudragit L30D-55 of the prescription amount, add an equal volume of water dissolving the prescription amount...
Embodiment 2
[0091] A compound telmisartan hydrochlorothiazide pharmaceutical composition, the compound telmisartan hydrochlorothiazide pharmaceutical composition described in every 1000
[0092] compound, its formula consists of:
[0093] Inner Tablet Prescription
[0094]
[0095] Outer Tablet Prescription
[0096]
[0097] Inner Tablet Coating Solution Prescription
[0098]
[0099] Preparation Process:
[0100] 1) Preparation of the inner layer tablet: After crushing hydrochlorothiazide through a 100-mesh sieve, place it in a rapid mixer with microcrystalline cellulose, crospovidone, and pregelatinized starch, and mix rapidly for 3-5 minutes, then add appropriate amount of water, Make soft material, pass through 20-mesh sieve, dry at 50±5℃, granulate with 20-mesh sieve, add sucrose fatty acid ester, and press into tablets.
[0101] 2) Inner layer tablet coating: Weigh the Eudragit L30D-55 of the prescription amount, add an equal volume of water dissolving the prescription...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com