Method for detecting ethanol and isopropanol in valsartan hydrochlorothiazide
A technology of hydrochlorothiazide and isopropanol, applied in the field of drug analysis, can solve problems such as uncontrolled residual amount, inability to apply valsartan hydrochlorothiazide, human injury, etc., and achieve stable and reliable results, good repeatability and durability, and quality assurance. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0108] Instruments and conditions: Shimadzu chromatography system, hydrogen flame ionization detector, chromatographic column: TRACE TR-WAX (30m×0.32mm, 0.25μm); detector temperature: 280°C; inlet temperature: 200°C; with nitrogen It is carrier gas; flow rate is 3ml / min; headspace sample injection, headspace temperature 100°C, headspace time 30min; initial column temperature: 45°C.
[0109] The temperature programmed elution settings were:
[0110]
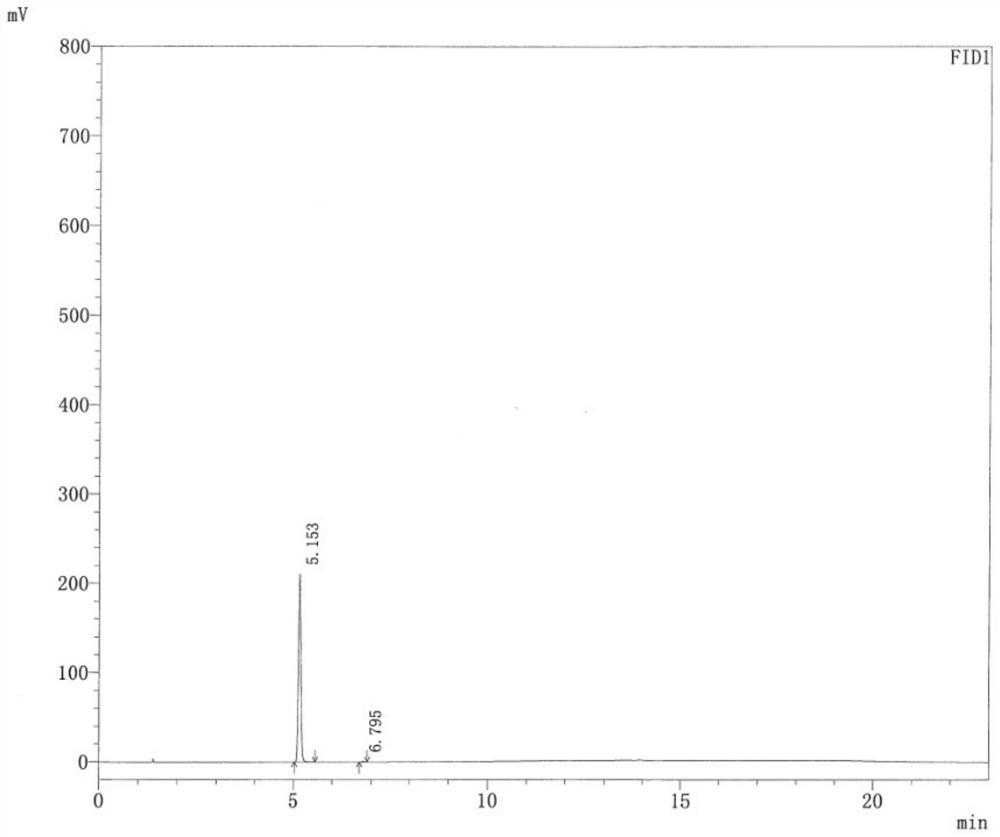
[0111] Determination: Take an appropriate amount of ethanol and isopropanol reference substance, add water to dissolve, prepare a reference substance solution containing 0.5 mg of ethanol and isopropanol per 1 ml, take 2 ml and place it in a 20 ml headspace bottle, seal it, and use it as a reference substance solution , determined according to the above-mentioned chromatographic conditions, and record the chromatograms. GC chart attached Figure 5 .
[0112] Depend on Figure 5 It can be seen that the retention time of etha...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com