Preparation method for compound losartan potassium-hydrochlorothiazide pharmaceutical composition
A technology of losartan potassium hydrochlorothiazide tablets and hydrochlorothiazide, which is applied in the field of preparation of losartan potassium hydrochlorothiazide tablets, can solve the problems affecting the release rate of amlodipine dissolution, the effect is not very obvious, and increase the volume ratio of auxiliary materials, etc., to achieve There is no obvious difference in loading capacity, good fluidity, and the effect of improving stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
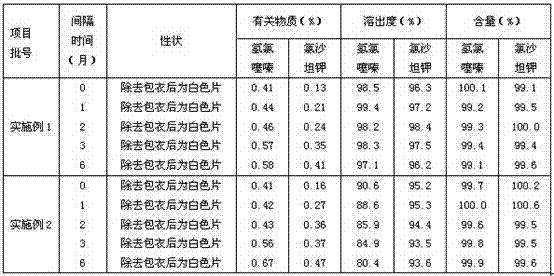
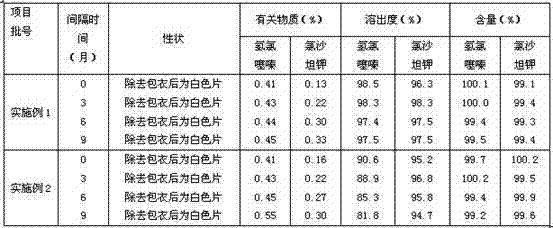
Embodiment 1
[0033] particle 1
[0035] Starch 150g
[0036] 10% starch slurry appropriate amount
[0037] particle 2
[0038] Hydrochlorothiazide 12.5g
[0039] Croscarmellose Sodium 10g
[0040] Lactose 150g
[0041] 5% povidone K30 solution appropriate amount
[0043] Makes 1000 pieces
[0044] The preparation steps are as follows:
[0045] 1) Sieve the prescription amount of losartan potassium, mix it evenly with starch, use 10% starch slurry to make a soft material with suitable hardness, use a 20-mesh sieve to granulate, and dry at 60°C to make the water content less than 5 %,spare;
[0046] 2) Pulverize the prescription amount of hydrochlorothiazide to a qualified particle size, and set aside;
[0047] 3) Mix the prescribed amount of hydrochlorothiazide, croscarmellose sodium, and lactose evenly, use 5% povidone K30 solution to prepare a soft material with suitable hardness, use a 20-mesh sieve to granulate, a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com