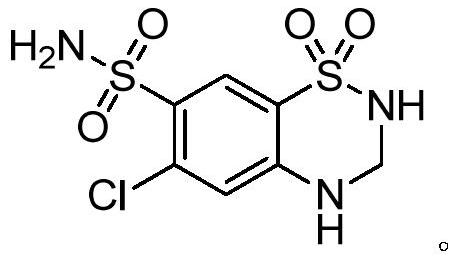
A kind of synthetic method of hydrochlorothiazide
A synthesis method and hydrochlorothiazide technology are applied in the synthesis field of hydrochlorothiazide, can solve problems such as difficult removal, high-salt waste water, and pollute the environment, and achieve the effects of mild process conditions, low purification difficulty, and improved yield and purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Preparation of 6-chloro-3,4-dihydro-2H-1,2,4-benzothiadiazine-7-sulfonamide-1,1-dioxide
[0063] Add sodium borohydride (2.5g, 67.6mmol, 2eq) into tetrahydrofuran (50mL), cool down to 0-5°C, add formic acid (6.2g, 135.2mmol, 4eq) dropwise at this temperature, stir for 1h after addition, cool down to -10°C, add the compound-chlorothiazide (10g, 33.8mmol, 1eq) at this temperature, keep it at -10~0°C for 3h, filter with suction, rinse the filter cake with a small amount of tetrahydrofuran (10mL), and obtain about 9.3g of white Solid (92.9% yield).
[0064] Product Characterization:
[0065] 1 HNMR (deuterated acetone, 400MHz): δ8.18(s,1H); 7.21(s,-NH,1H); 7.07(s,1H); 6.81(s,SO 2 NH,1H); 6.68(s,SO 2 NH 2 ,2H); 4.95-4.94(d,2H);
[0066] ESI-MS(m / z):297[M-H] - .
Embodiment 2
[0068] Preparation of 6-chloro-3,4-dihydro-2H-1,2,4-benzothiadiazine-7-sulfonamide-1,1-dioxide
[0069] Add potassium borohydride (3.65g, 67.6mmol, 2eq) into methanol (50mL), cool down to 0-5°C, add acetic acid (8.11g, 135.2mmol, 4eq) dropwise at this temperature, and stir for 1-2h after addition. Cool down to -10°C, add the compound-chlorothiazide (10g, 33.8mmol, 1eq) at this temperature, keep it at -10~5°C for 4h, filter with suction, rinse the filter cake with a small amount of methanol (10mL), and obtain about 9.4 g white solid (93.9% yield).
[0070] Product Characterization:
[0071] 1 HNMR (deuterated acetone, 400MHz): δ8.18(s,1H); 7.21(s,-NH,1H); 7.07(s,1H); 6.81(s,SO 2 NH,1H); 6.68(s,SO 2 NH 2 ,2H); 4.95-4.94(d,2H);
[0072] ESI-MS(m / z):297[M-H] - .
Embodiment 3
[0074] The difference with embodiment 1 is that the consumption of sodium borohydride is different, specifically as follows:
[0075] Add sodium borohydride (1.3g, 33.9mmol, 1eq) into tetrahydrofuran (50mL), cool down to 0-5°C, add formic acid (3.1g, 67.6mmol, 2eq) dropwise at this temperature, stir for 1h after addition, cool down to -10°C, add the compound-chlorothiazide (10g, 33.8mmol, 1eq) at this temperature, keep it at -10~0°C for 3h, filter with suction, rinse the filter cake with a small amount of tetrahydrofuran (10mL), and obtain about 7.8g of white Solid (78.0% yield).
[0076] Product Characterization:
[0077] 1 HNMR (deuterated acetone, 400MHz): δ8.18(s,1H); 7.21(s,-NH,1H); 7.07(s,1H); 6.81(s,SO 2 NH,1H); 6.68(s,SO 2 NH 2 ,2H); 4.95-4.94(d,2H);
[0078] ESI-MS(m / z):297[M-H] - .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com