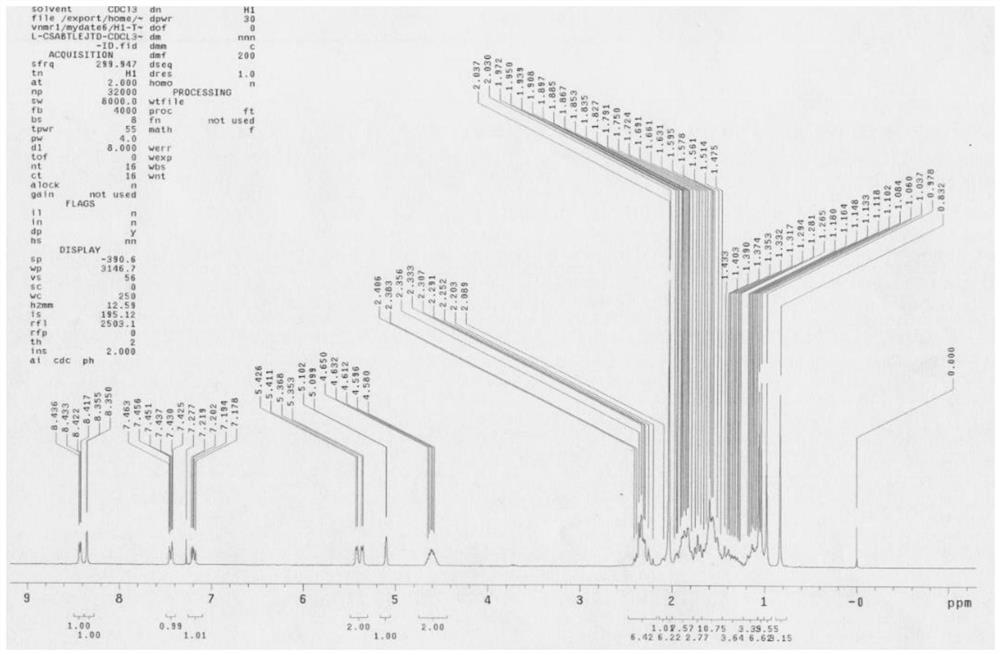
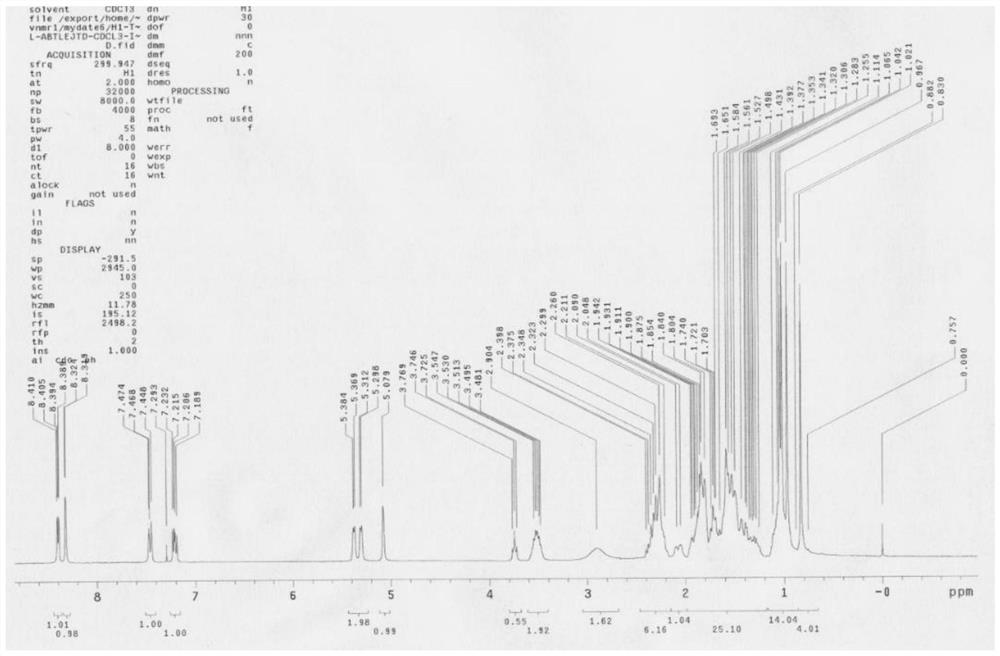
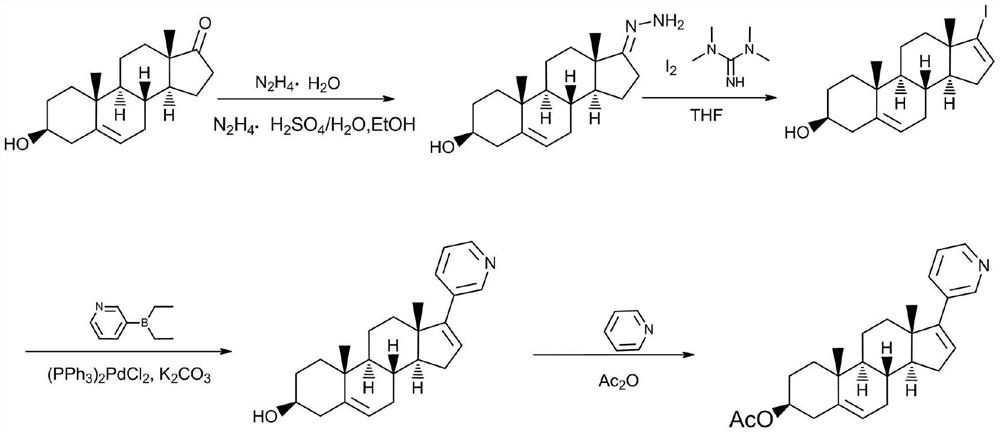
Method for separating and purifying abiraterone and dimer of the abiraterone
A technology of abiraterone and dimer, which is applied in the field of biomedicine, can solve the problems of the uncertainty of the purity of the final product, the absence of high-purity abiraterone dimer, and the unsatisfactory removal effect of abiraterone dimer. To achieve the effect of reducing the difficulty of refining
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] A method for separating and purifying abiraterone and dimers thereof, comprising the following steps:
[0024] (1) Dissolve 98g (0.246mol) 17-iodo-androst-5,16-diene-3β-ol in 1.1LTHF in a 2L reaction flask, add 1.73g (0.0025mol) bistriphenylphosphine dichloride Palladium chloride and 43.35g (0.295mol) diethyl (3-pyridyl) borane, then in this reaction solution, add the K of 2M 2 CO 3 500ml of aqueous solution was heated to reflux for 4 days, the residual amount of 17-iodo-androst-5,16-dien-3β-ol was monitored by HPLC <0.5%, and the reaction was stopped to obtain the abiraterone reaction solution.
[0025] (2) Cool down to 15°C, continue to stir for 2h at this temperature, filter to obtain wet product filter cake I3.2g
[0026] (3) Put filter cake I and 0.32g gac into 32ml tetrahydrofuran and heat up and reflux for 30min, heat filter to obtain filtrate II and filter cake II;
[0027] (4) The filtrate II was concentrated to dryness under reduced pressure to obtain a res...
Embodiment 2
[0030] A method for separating and purifying abiraterone and dimers thereof, comprising the following steps:
[0031] (1) The process of obtaining the abiraterone reaction solution is the same as in Example 1.
[0032] (2) Cool down to 20°C, continue to stir for 2h at this temperature, filter to obtain wet product filter cake I2.9g
[0033] (3) Put filter cake I and 0.29g activated carbon into 32ml THF and heat up to reflux for 30min, heat filter to obtain filtrate II and filter cake II;
[0034] (4) The filtrate II was concentrated to dryness under reduced pressure to obtain 2.3 g of residue.
[0035] (5) Add 4.6ml of tetrahydrofuran to the obtained residue, raise the temperature to reflux, stir to dissolve, drop to 10°C, stir and crystallize for 3h, and filter to obtain 1.9g of the target product abiraterone dimer with a purity of 99.38%.
Embodiment 3
[0037] A method for separating and purifying abiraterone and dimers thereof, comprising the following steps:
[0038] (1) The process of obtaining the abiraterone reaction solution is the same as in Example 1.
[0039] (2) Cool down to 18°C, continue to stir for 3h at this temperature, filter to obtain wet product filter cake I2.7g
[0040] (3) Put filter cake I and 0.27g gac into 274ml tetrahydrofuran, heat up and reflux for 30min, heat filter, get filtrate II and filter cake II;
[0041] (4) The filtrate II was concentrated to dryness under reduced pressure to obtain 2.3 g of residue.
[0042] (5) Add 11.5ml of tetrahydrofuran to the obtained residue and raise the temperature to reflux, stir to dissolve, drop to 5°C, stir and crystallize for 2.5h, and filter to obtain 1.86g of the target product abiraterone dimer with a purity of 99.56%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com